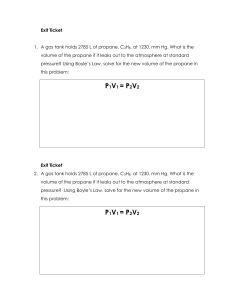
Percent Composition
advertisement

Percent Composition % Composition from the Formula • % mass = mass of element in 1 mole of compound molar mass of compound • Then multiply by 100 to get % • Propane (C3H8) is one of the compounds obtained from petroleum. Calculate the percent composition of propane. • Need to find mass of each element in 1 mole of the compound & mass of the 1 mole of the compound • Mass of C in one mole of C3H8= 36.033 g • Mass of H in one mole of C3H8= 8.064 g • Molar mass of C3H8= 44.097 g • % of C = mass of C mass of propane x 100 (36.033 g/44.097 g) x 100 = 81.713% • % of H = mass of H mass of propane x 100 (8.064 g/44.097 g) x 100 = 18.29 % • Results should add up to approximately 100%. • If not, you did something wrong! Using % composition • Can determine how much of a substance can be produced. • For example, how many grams of hydrogen are contained in a 100 g sample of propane? • How many grams of hydrogen are contained in a 39.0 g sample of propane? • How many grams are carbon? Examples • Determine the percent composition of Al(PO4). Lab Directions • Complete “Day 1” Instructions • Safety! • Place a sheet of paper with your names underneath your beaker on back lab table