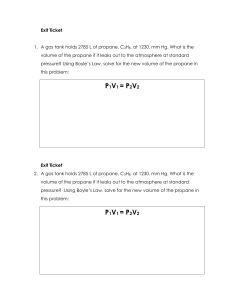
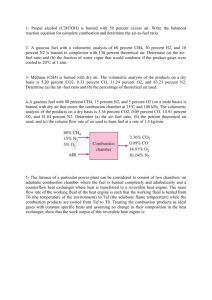
Propane C3H8 is burned. What will be the mole number of Oxygen in the combustion equation with 50% excess air? The correct answer is: 7.5 Determine the stoichiometric gravimetric air-fuel ratio for the complete combustion of n-Butane (C4H10). The correct answer is: 15.45 kg air/kg fuel Propane C3H8 is burned. What will be the mole number of Nitrogen in the combustion equation under theoretical air? The correct answer is: 18.8 10kg of flue gas formed per kg of fuel burned in the combustion of Butane C4H10 What is the A/F? The correct answer is: 9 Propane C H is burned with 50% excess air. What will be the equivalence ratio? (Round off your answer to nearest two decimal places) The correct answer is: 0.67 Determine the actual gravimetric air-fuel ratio for the complete combustion of n-Butane (C4H10 ) with 50% excess air. The correct answer is: 23.12 kg air/kg fuel Propane (C3H8 ) is burned with 30% excess air. Determine the stoichiometric molar A/F ratio. The correct answer is: 23.8 mol air/mol fuel Propane C3H8 is burned. What is the stoichiometric gravimetric air-fuel ratio? The correct answer is: 15.66 kg air/kg fuel Propane (C3H8 ) is burned with 30% excess air. Determine the actual molar A/F ratio. The correct answer is: 30.94 mol air/mol fuel Propane C H is burned with 50% excess air. What is the actual gravimetric air-fuel ratio? The correct answer is: 23.5 kg air/kg fuel