Chapter 16: Glycolysis
advertisement

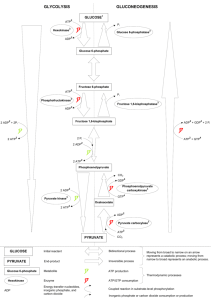
Chapter 14 1 Takusagawa’s Note© Chapter 14: Glycolysis ATP from a piece of bread 1. α-Amylose in a bread is partially digested in mouse with saliva amylase to glucose, maltose and short amylose. 2. Maltose and amylose are further digested in small intestine by the pancreatic α-amylase to glucose. 3. Glucose is absorbed through a brush border cell into bloodstream. 4. Some of glucoses are transported into liver and stored as glycogen, and the others are entered into heart. 5. When the glucose concentration in blood is low, glucose from glycogen in liver is put into bloodstream. 6. Air is breathed into lung. 7. Hb in blood becomes HbO2, then goes back to heart. 8. Glucose and HbO2 are transported to the surface of a muscle cell in arm through bloodstream. 9. Glucose enters the cell through a glucose transporter. 10. O2 from HbO2 enters the cell by a simple diffusion. 1 Chapter 14 2 2 Takusagawa’s Note© Takusagawa’s Note© 3 Chapter 14 Aerobic pathway HbO2 Cell Bloodstream Glucose glucose transport Cytosol Glucose O2 2ATP Glycolysis + 2NADH NAD+ 2Pyruvate Oxidative phosphorylation H+ + H V ADP H+ e- I IV e- III ATP H+ NAD+ NADH H2O O2 NAD+ 2Py 2NADH 36ATP ADP ATP ADP ADP ATP translocator 2Acety-CoA 6NADH 2FADH2 2ATP Mitochondrion 4CO2 3 Citric acid cycle 2CO2 pyruvate-H+ transport Takusagawa’s Note© 4 Chapter 14 1. THE GLYCOLYTIC PATHWAY Glycolysis O Glucose 2ADP + 2Pi 2NAD + Fructose-1,6bisphosphate 2ATP 2NADH 2Pyruvate Aerobic oxidation Anaerobic homolactic fermentation Citric Acid Cycle 2NADH 2NADH 2NADH 2NAD Anaerobic alcoholic fermentation 6O2 Oxidative phosphorylation + 2NAD 2Lactate 2NAD + + 6CO2 + 6H2O 2CO2 + 2Ethanol Basic information - Glucose enters most cells by a specific carrier that transports it from the exterior of cell into the cytosol. - The enzymes of glycolysis are located in the cytosol. - Glycolysis converts glucose to two C3 units (pyruvate), and released free energy is used to synthesize ATP from ADP and Pi. Post glycolysis pathways - Two major pathways of post glucose metabolism: - Aerobic oxidation --- 2Pyruvate → 6CO2 + 6H2O (and 30 ATP) - Anaerobic fermentation - Homolactic in muscle --- 2Pyruvate → 2Lactate (no ATP) - Alcoholic in yeast --- 2Pyruvate → 2CO2 + 2EtOH - Overall glycolysis is shown in Fig. 16-3. 4 Chapter 14 5 5 Takusagawa’s Note© Chapter 14 6 Takusagawa’s Note© Glycolysis may be considered to occur in two stages - Stage I: Glucose is phosphorylated and cleaved to two trioses, glyceraldehyde-3phosphate (GAP) [Step-1 to 5]. 2ATP Glucose ⎯ ⎯⎯ ⎯→ 2GAP --- (2ATPs are used) - Stage II: Two glyceraldehyde-3-phosphates are converted to two pyruvates [Step-6 to 10]. 2GAP ⎯⎯→ 2Pyruvate --- (4ATP and 2NADH are generated) - Thus, net profit is 2ATPs and 2NADH per glucose. Overall reaction is: Glucose + 2ADP + 2NAD+ + 2Pi → 2pyruvate + 2ATP + 2NADH + 2H2O + 4H+ Oxidizing power of NAD+ must be recycled - The number of NAD+ molecules in a cell is limited. Thus in order to continue glycolysis, NADH must be oxidized to NAD+. 1. Under anaerobic condition in muscle: Pyruvate → lactate and NADH → NAD+ 2. Under anaerobic condition in yeast: Pyruvate → ethanol + CO2, and NADH → NAD+ + ΔH 3. Under aerobic condition: Pyruvate → CO2 + H2O, and NADH → NAD+ + 3ATP - In anaerobic glycolysis, the free energy of oxidation (NADH → NAD+) is wasted as heat (ΔH). - In aerobic glycolysis, NADH is a “high-energy” compound, and produces 3ATP per NADH. 2. THE REACTIONS OF GLYCOLYSIS A. Glucose → Glucose-6-phosphate (G6P), First ATP Utilization Enzyme: Hexokinase (HK) Reaction: Transfer phosphoryl group ΔG°′ = -20.9 kJ/mol; ΔG = -27.2 kJ/mol (Physiological condition) Reaction mechanism - C6-OH group of glucose nucleophilic attacks on the γ-phosphate of an Mg2+-ATP complex. 6 Chapter 14 7 Takusagawa’s Note© Note: Kinase - an enzyme that transfers phosphoryl groups between ATP (actually ATP-Mg2+) and a metabolite (glucose in this case). - The phosphoryl group acceptor for a specific kinase is identified in the prefix of the kinase name. Thus, the name of enzyme is glucokinase. But the enzyme is not very specific to glucose, i.e., the enzyme transfers phosphate group to several hexoses. Therefore the name of this enzyme is hexokinase. Hexokinase changes its conformation upon the binding of substrate (glucose) Question: Why does hexokinase catalyze the transfer of a phosphoryl group from ATP to glucose to yield G6P, but not to water to yield (ADP + Pi) ? C6-OH + ATP → ADP + C6-O-PO32- ---- (1) ΔG = -13.8 kJ/mol H-OH + ATP → ADP + Pi ----------------- (2) ΔG = -30.5 kJ/mol - A water molecule is small enough to fit into the enzyme active site where the C6-OH of glucose fits. - Reaction (2) (ΔG = -30.5 kJ/mol) is more exergonic than reaction (1) (ΔG = -13.8 kJ/mol). See Table 15-3. - Nevertheless Reaction (1) is 40,000 time faster than reaction (2). Answer: A glucose induces a large conformational change in hexokinase. This movement places the ATP in close proximity to the C6-OH group of glucose and excludes water from the active site. H2O does not cause the conformational changes, but glucose does cause the large conformational changes in the enzyme structure. 7 Chapter 14 B. 8 Takusagawa’s Note© Glucose 6-phosphate (G6P) to Fructose 6-phosphate (F6P) Enzyme: Phosphoglucose isomerase (PGI) Reaction: Isomerization of an aldose to ketose ΔG°′ = +2.2 kJ/mol; ΔG = -1.4 kJ/mol Reaction mechanism - The reaction requires ring opening, followed by isomerization, and subsequent ring closure. 1. An acid (ε-amino group of Lys) donates a proton to O5 to open the ring. 2. A base (carboxylate of Glu) abstracts the acidic proton from C2. 3. The proton is replaced on C1. 4. The proton on O5 is returned to the acid (Lys) and the O5 nucleophilic attacks on C2 to close the ring. Lys 8 9 Chapter 14 C. Takusagawa’s Note© Fructose 6-phosphate (F6G) to Fructose-1,6-bisphosphate (FBP) , Second ATP Utilization Enzyme: Phosphofructokinase (PFK) Reaction: Transfer phosphoryl group ΔG°′ = -17.2 kJ/mol; ΔG = -25.9 kJ/mol Reaction mechanism - Similar to the hexokinase reaction. - PFK is allosterically enhanced by AMP, and allosterically inhibited by ATP and citrate. D. Fructose-1,6-bisphosphate to two trioses, Glyceraldehyde-3-phosphate (GAP) and Dihydroxyacetone phosphate (DHAP) 2- O3POH2C CH2PO O 2- HO 4 OH 3 OH Ring FBP Enzyme: Aldolase Reaction: Aldol cleavage ΔG°′ = +22.8 kJ/mol; ΔG = -5.9 kJ/mol - Note that aldol cleavage between C3 and C4 of FBP requires a carbonyl at C2 and hydroxyl at C4. Aldol cleavage of F6P would produce equal carbon chain length (two C3), but aldol cleavage of G6P would produce unequal carbon chain length (C2 and C4). Therefore isomerization of G6P to F6P is required. 9 Chapter 14 10 Takusagawa’s Note© Reaction mechanism 1. The active site has a neutral Lys (-NH2) and negatively charged Tyr (-O-). 2. Schiff base formation between the enzyme (Lys) and FBP. 3. Tyr abstracts the proton from FBP, thus the C3-C4 bond is cleaved (aldol cleavage). 4. Protonation on C3 from Tyr-OH, and tautomerization of Schiff base. 5. Hydrolysis of the Schiff base. 10 Takusagawa’s Note© 11 Chapter 14 Two classes of aldolase 1. The enzyme in animals and plants forms a Schiff base, i.e., has a neutral Lys-NH2 to form Schiff base with O=C of FBP. 2. The enzyme in fungi, algae and some bacteria contains Zn2+ or Fe2+ ion, i.e., these metal ions polarize the C=O of FBP instead of Schiff base formation. Aldolase reaction is stereospecific - The enzyme distinguishes the pro-S and pro-R hydrogens at the C3, i.e., the proton from Tyr is attached at the pro-S site of C3. - This stereospecific reaction is confirmed by the reverse reaction using DHAP and GAP. - If the protons at C3 of DHAP were transferred randomly to Tyr of the enzyme and to the carbonyl oxygen at C4 of GAP, the reverse reaction would produce 4 different products as shown below. H 1 CH2 OPO 322C HO O 4C H 5C OH 26CH2 OPO 3 + CH2 3 GAP DHAP HO 1CH2 OPO 32- 21CH2 OPO 3 2C O 2 C H H OH H OH H 3 H 4C H C 5 26 CH2 OPO 3 D-Fructose 1,6-bisphosphate - 1CH2 OPO 32- 21CH2 OPO 3 2C O 2C H 3C HO 4C C O C OH HO C H C OH H C OH HO 4C H 5C 3 4 O 3 OH O OH H 26 CH2 OPO 3 26 CH2 OPO 3 H 5C OH 26 CH2 OPO 3 D-Psicose 1,6-bisphosphate D-Tagatose 1,6-bisphosphate D-Sorbose 1,6-bisphosphate 5 However, only FBP is formed. This indicates that both DHAP and GAP must bind to the enzyme with correct orientation before taking place the reaction. 11 Chapter 14 12 Takusagawa’s Note© E. Dihydroxyacetone phosphate (DHAP) to Glyceraldehyde-3-phosphate (GAP) Enzyme: Triose phosphate isomerase (TIM) Reaction: Isomerization (aldose to ketose) ΔG°′ = +7.9 kJ/mol; ΔG = +4.4 kJ/mol Reaction mechanism 1. Glu-165 interacts with the proton on C2 of GAP, and the proton of His-95 interacts with C1=O to form GAP:TIM Michaelis complex. 2. At the first transition state, these two protons participate in the low-barrier hydrogen bonds (very short hydrogen bonds). 3. The protons are moved to the carbonyl oxygen of Glu-165 and to the carbonyl oxygen of GAP. 4. At the second transition state, Glu-165 donates the proton to C1 of GAP, and GAP donates the proton of O2-H to His-95 through the low-barrier hydrogen bonds. 5. After the proton transfer, DHAP:TIM Michaelis complex is formed. 12 Chapter 14 13 Takusagawa’s Note© TIM is a perfect enzyme --- Why? - The catalytic rate (kcat/KM = 109 M-1s-1) of TIM is near the rate of molecular collision and the rate of molecular diffusion into cells, Therefore, TIM is called a “perfect enzyme”. - TIM converts GAP to DHAP instantly. For this reason, - The equilibrium constant, K = [GAP]/[DHAP] = 4.73 x 10-2 is very low, i.e., [DHAP] >> [GAP]. - You might think that TIM should catalyze DHAP → GAP instead of GAP → DHAP, since the substrate of the next step of glycolysis is GAP but not DHAP. GAP inhibits the previous aldolase reaction. Therefore it is important to keep low [GAP] for proceeding the glycolysis pathway. When GAP is utilized in the succeeding reaction, more DHAP is very quickly converted to GAP. F. Glyceraldehyde 3-phosphate (GAP) to 1,3-Bisphophoglycerate (1,3-BPG), First “HighEnergy” Intermediate Formation Enzyme: Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) Reaction: Oxidation and phosphorylation ΔG°′ = +6.7 kJ/mol; ΔG = -1.1 kJ/mol 13 14 Chapter 14 Takusagawa’s Note© Reaction mechanism - The active site has the essential sulfhydryl group (-SH), a base residue (-B:) and cofactor NAD+. 1. GAP binds to the enzyme. 2. The base amino acid residue abstracts the proton from -SH, and thus the activated -S- attacks on the carbonyl carbon to form thiohemiacetal intermediate. 3. By transferring the proton to NAD+, the thiohemiacetal becomes an acyl thioester. 4. NADH is replaced with another NAD+. 5. A phosphate ion enters into the active site and its nucleophilic attack on the carbonyl carbon of acyl thioester forms 1,3-bisphosphoglycerate. The -S receives a proton from the base amino acid residue, and the product is released. - The enzyme is inhibited by iodoacetate, indicating that the active site contains Cys residue. Acyl phosphates are compounds with high phosphate group-transfer potential. O High energy bond R C OPO3 2- 14 Chapter 14 15 Takusagawa’s Note© G. 1,3-Bisphosphoglycerate (1,3-BPG) to 3-Phosphoglycerate (3PG): First ATP Generation Enzyme: Phosphoglycerate kinase (PGK). Reaction: Phosphorylation ΔG°′ = -18.8 kJ/mol; ΔG = ~0 kJ/mol Reaction mechanism - Nucleophilic attack of the terminal phosphoryl oxygen of ADP on the C1 phosphorus atom of 1,3-BPG forms the reaction product. - Note that enzyme name is not “bisphosphoglycerate kinase”, since 3-PG but not BPG receives the Pi from ATP by the reverse reaction. - This reaction is large exergonic (ΔG°′ = -18.8 kJ/mol) and pulls the previous endergonic reaction (ΔG°′ = +6.7 kJ/mol). ΔG°′ kJ/mol GAP + Pi + NAD+ → 1,3-BPG + NADH +6.7 1,3-BPG + ADP → 3PG + ATP -18.8 ⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯ GAP + Pi + NAD+ + ADP → 3PG + NADH + ATP -12.1 H. 3-Phosphoglycerate (3PG) to 2-Phosphoglycerate (2PG) 15 Chapter 14 16 Takusagawa’s Note© Enzyme: Phosphoglycerate mutase (PGM) Reaction: Intramolecular phosphoryl group transfer ΔG°′ = +4.7 kJ/mol; ΔG = -0.6 kJ/mol Reaction mechanism 1. 3PG binds to the phosphorylated enzyme (E-His-PO3-). 2. The PO3- is transferred to the substrate to form 2,3-BPG:E complex. 3. The PO3- attached on the O3 is transferred to the enzyme, and 2PG is released. 16 Chapter 14 - 17 Takusagawa’s Note© 2,3-Bisphosphoglycerate (2,3-BPG) is an intermediate of the reaction (step-5 in the previous page). Thus, glycolysis influences oxygen transport (review of Chapter-9). BPG binds into the central cavity of the Hb molecule and stabilizes the T-form (deoxy-Hb). Thus, high [BPG] in blood reduces the oxygen affinity of Hb molecules. Hexokinase deficient results in low [3PG], i.e., low [G6P]→→→ low [3PG]→ low [2,3-BPG], thus increase Hb O2 affinity. (more HbO2) Pyruvate kinase deficient results in accumulation of [2PG], i.e., high [PEP] →high [2PG] → high [2,3-BPG], thus decrease Hb O2 affinity. (more Hb) I. 2-Phosphoglycerate (2PG) to Phosphoenolpyruvate (PEP) Enzyme: Enolase Reaction: Dehydration ΔG°′ = -3.2 kJ/mol; ΔG = -2.4 kJ/mol 17 Chapter 14 18 Takusagawa’s Note© Reaction mechanism - The enzyme forms a complex with Mg2+ before the substrate is bound. - Fluoride ion (F-) inhibits this process, since F- forms a complex with bound Mg2+ in the active site, and block the substrate binding. - A water molecule is in the active site, which hydrogen-bonds to two Glu residues (?). 1. The water molecule bound to the two carboxylates of Glu residues abstracts a proton at C2, and thus the carboanion is formed (rapid reaction). The abstracted proton is readily exchanged with a proton in the solvent. 2. Slow elimination of Mg2+-stabilized OH at C3 produces a phosphoenolpyruvate (PEP) and a water molecule. 18 Takusagawa’s Note© 19 Chapter 14 J. Phosphoenolpyruvate (PEP) to Pyruvate Enzyme: Pyruvate kinase Reaction: Hydrolysis to ATP synthesis ΔG°′ = -23.0 kJ/mol; ΔG = -13.9 kJ/mol Reaction mechanism - Monovalent (K+) and divalent (Mg2+) cations are required. 1. A nucleophilic attack of the ADP βphosphoryl oxygen atom on the phosphorus atom of PEP forms ATP and enolpyruvate. 2. A tautomerization is taken place to form a pyruvate from a enolpyruvate. This process is quite exergonic (ΔG° = -31.4 kJ/mol) and thus pulls the step-1 reaction (ATP synthesis). Why is 2PG not directly converted to pyruvate? - ΔG°′ of hydrolysis of 2PG is only -17.6 kJ/mol which is not enough to synthesize ATP from ADP (ΔG°′ = -30.5 kJ/mol). - Dehydration of 2PG results in the formation of “high-energy” compound (PEP) capable of ATP synthesis whose ΔG°′ of the hydrolysis is -61.9 kJ/mol. - Thus, A simple conversion of 2PG → pyruvate does not produce a sufficient energy to synthesize ATP, but the (2PG →PEP → pyruvate) conversion produces enough energy to synthesize ATP. E 2PG E ΔG = -17.6 Glycerate Pyruvate 2PG ΔG = -61.9 19 Pyruvate Chapter 14 20 Takusagawa’s Note© 3. FERMENTATION: ANAEROBIC FATE OF PYRUVATE - Amount of NAD+ in a cell is limited. Thus, NADH produced by GAPDH must be recycled in order to continue glycolysis. - Under aerobic condition, NADH is re-oxidized by sending electrons into the mitochondria. - Under anaerobic condition, the NAD+ is replenished by the reduction of pyruvate by two processes: - Homolactic fermentation (in muscle) - Alcoholic fermentation (in yeast) A. Homolactic fermentation Reaction mechanism 1. The substrate is tightly connected to the enzyme by salt bridges between the COO- of pyruvate and the guanidinyl group of Arg-171. 2. The carbonyl oxygen of pyruvate receives a proton from His-195, and in the meantime, the pro-R H of NADH is directly transferred to pyruvate (direct hydride transfer). 20 - This reaction is absolute stereo-specificity --- The pro-R H of NADH is transferred to lactate. On the other hand, in the glyceraldehyde 3-phosphate dehydrogenase (GAPDH), the pro-S H of NADH is transferred to GAP (see pages 12-13). This occurs because the orientation of the nicotinamide ring in the LDH structure differs by 180° of that in the GAPDH structure (also see the next page). Pro-R Pro-S Pro-S Pro-R O H2N H C - O C 180° rotation NH2 N R GAPDH LDH R GAPDH LDH - H H H N - Takusagawa’s Note© 21 Chapter 14 Overall process of anaerobic glycolysis in muscle is: Glucose + 2ADP + 2Pi → 2 lactate + 2ATP + 2H2O + 2H+ Much of the lactates in the muscle cells are carried by blood to liver, and are reconverted to glucose. Muscle fatigue and soreness are caused by the accumulation of glycolytically generated acid (H+), but not lactate. 21 Chapter 14 22 22 Takusagawa’s Note© Takusagawa’s Note© 23 Chapter 14 B. Alcoholic Fermentation - is a two step reaction. 1. Decarboxylation of pyruvate to form acetaldehyde. 2. Reduction to ethanol by NADH. O H3C O C C O Pyruvate 1 pyruvate decarboxylase NAD+ NADH CO2 H3C O 2 H alcohol dehydrogenase C Acetaldehyde OH H3C H Ethanol Decarboxylation - Pyruvate decarboxylase requires a cofactor thiamine pyrophosphate (TPP). 23 C H Chapter 14 24 Takusagawa’s Note© Reaction mechanism 1. TPP becomes the ylid form by releasing the acidic proton at C2. The nucleophilic attack of ylid form of TPP on the carbonyl carbon of pyruvate forms the transition complex. 2. After releasing the CO2, the carbanion is stabilized by taking resonance form. 3. A proton in solvent is attached on the carbanion (protonation). 4. Acetaldehyde is released from the ylid TPP. - Beriberi is a thiamine (vitamin B1) deficiency disease. Reduction by NADH - NAD+ is regenerated by reduction of acetaldehyde to ethanol. 24 Chapter 14 25 Takusagawa’s Note© Reaction mechanism 1. The substrate (acetaldehyde) coordinates to Zn2+ which is in the active site of alcohol dehydrogenase. 2. The pro-R H of NADH is directly transferred to the carbonyl carbon of acetaldehyde from the re-side of the molecule (direct hydride transfer). Both homolactic and alcoholic fermentation have the same function - That is “anaerobic regeneration of NAD+”, in order to continue glycolysis quickly to produce ATP molecules by glycolysis. C. - - Energetic of Fermentation For homolactic fermentation: Glucose → 2 lactate + 2H+ ΔG°′=-196 kJ/mol ΔG°′=-235 kJ/mol For alcohol fermentation: Glucose → 2 ethanol + 2CO2 Each of these reactions produce 2ATP (useful energy source of biological system), which requires ΔG°′ = +61 kJ/mol. The efficiency of fermentations are: 61/196 = 0.31 for homolactic fermentation. 61/235 = 0.26 for alcohol fermentation. - The rest is dissipated as heat. Glycolysis is used for rapid ATP production. - ATP production of anaerobic glycolysis is ~100 times faster than that of oxidative phosphorylation (aerobic pathway). - Thus, tissues such as muscle consuming ATP rapidly regenerate it almost entirely by anaerobic glycolysis. - Since the end product, lactate, is aerobically regenerated to glucose in liver, the homolactic fermentation does not really waste glucose. 25 Chapter 14 - 26 Takusagawa’s Note© Red muscle fiber contains a large amount of mitochondria which produce ATP by oxidative phosphorylation (aerobic pathway). White muscle fiber contains less amount of mitochondria, indicating that ATP is generated by anaerobic fermentation (anaerobic pathway). 5. CONTROL OF METABOLIC FLUX - ATP consumptions at resting and vigorous exertion times are quite different. - But the [ATP] is nearly constant (steady state). On the other hand, the flux rate of glycolysis is increased by 100-folds at vigorous exertion. - How does our body control the metabolic flux? Maybe: All enzymes involved in glycolysis are activated, Slow or a few some key enzymes are activated. If this is the case, which enzymes are activated and how? Fast - How is a signal of the [ATP] change delivered to the glycolysis flux change? A. Flux generation - The flux of metabolites, J, through each reaction step is: J = vf - v r where vf and vr are the rate of the forward reaction and reverse reaction, respectively. - At equilibrium, J = 0, although vf and vr are not zero. Thus it is impossible to control the flux at near equilibrium. - For irreversible step, J = vf. The flux is controlled by the specific enzyme activity. The rate of enzymatic reactions respond to changes in flux - Let us consider how a constant flux is maintained throughout a metabolic pathway. - A simple steady state pathway is: - In the steady state, the [A] and [B] are apparently constant. 26 - - Takusagawa’s Note© 27 Chapter 14 If the flux rate of the rate-determining step increases by ΔJ, ∴ΔJ = Δvf The fractional change in flux (ΔJ/J) through the rate determining step is: vf ΔJ Δv f Δv f v f Δv f = = ⋅ = ⋅ J J vf J v f v f − vr ( ) ↑ Fractional change in vf - Michaelis-Menten equation of the A ←⎯→ B reaction is v f = - In physiological condition, KM >> [A], thus, v f = - Thus, Δv f vf = Δ[ A] [ A] f Vmax [ A] K M + [ A] f Vmax [ A] V f Δ[ A] and Δv f = max KM KM vf ΔJ Δv f Δ[ A] v f Therefore, = ⋅ = [ A] v f − vr J v f v f − vr ( ) ( ) Conclusion 1. Irreversible condition: v r → 0, vf (v f − v r ) →1 Thus, ΔJ Δ[ A] = J [ A] The fractional change in flux is proportional to the fractional change in its substrate concentration. Therefore, a significant increase of flux requires a significant increase of the substrate concentration --- slow response. vf ΔJ Δ[ A] 2. Near equilibrium condition: vr → v f , → ∞ Thus, = ⋅ X ( X → ∞) J (v f − vr ) [ A] Since we assume that [A] is constant, Δ[A] must be very small. Therefore, a very small increase of substrate concentration can respond to a significant flux change (ΔJ) --- fast response. For this reason, most metabolic reactions in a series of sequential pathway are near the equilibrium, and thus have the same flux. The flux through a pathway is controlled at its rate determining step - In irreversible reaction, products are removed before substrates reach the equilibrium. This indicates: 1. The rate of irreversible reaction must be much slower than reversible reaction. 2. Therefore the irreversible reactions are rate determining steps. 3. The reaction must be exergonic, i.e., ΔG < 0 (spontaneous reaction). - The flux through the rate-determining step of a pathway may altered by several mechanisms. 1. Allosteric control 2. Covalent modification 3. Substrate cycle (futile cycle) 4. Genetic control (Control of enzyme production from transcription and translation stages). B. Control of glycolysis in muscle 27 - - Takusagawa’s Note© 28 Chapter 14 The flux rates of the near equilibrium reactions are very sensitive to changes in substrate concentrations. Thus it is not suitable to activate the enzymes in the near equilibrium reactions in order to increase significantly the flux rate (a large ΔJ gives a small Δ[A]). A large flux change must be accomplished by changing the flux rates of non-equilibrium reactions (a large ΔJ gives a large Δ[A]). Thus, non-equilibrium reactions are the candidates for the flux-control points. Three reactions with large -ΔG in muscle are catalyzed by: Hexokinase (HK); Phosphofructokinase (PFK); Pyruvate kinase (PK) These three reactions are non-equilibrium. Others are near equilibrium at physiological condition. Standard free energy changes (ΔG°′) and physiological free energy changes (ΔG) in heart muscle of the reactions of glycolysis. ΔG°′ = Standard free energy change ΔG = Actual physiological free energy change ΔG°′ ≈ ΔG (but some exceptions) Reaction 1 2 3 4 5 6+7 8 9 10 Enzyme HK PGI PFK Aldolase TIM GAPDH + PGK PGM Enolase PK Standard condition ΔG°′ (kJ/mol) -20.9 +2.2 -17.2 +22.8 +7.9 -16.7 +4.7 -3.2 -23.0 Physiological condition ΔG (kJ/mol) -27.2 -1.4 -25.9 -5.9 +4.4 -1.1 -0.6 -2.4 -13.9 Phosphofructokinase (PFK) is the major flux-controlling enzyme of glycolysis in muscle - Although the -ΔG of hexokinase is largest, the G6P from glycogen does not require the hexokinase. Thus, hexokinase cannot control the flux of glycolysis. - Some effectors of the non-equilibrium enzymes of glycolysis. Enzyme Inhibitors Activators Hexokinase G6P* PFK ATP, citrate*, PEP* ADP, AMP, cAMP, FBP, [F2,6P], F6P, NH4+, Pi PK (muscle) ATP* * Feedback inhibitirs (products). PFK has two conformational states, T (inactive) and R (active). 28 Chapter 14 29 Takusagawa’s Note© - ATP is both a substrate and an allosteric inhibitor of PFK. PFK has two ATP binding sites in each subunit --- one is the catalytic site and the other is allosteric site. - ATP (weak inhibitor) binds to the T form, and stabilizes it. Thus the equilibrium T ↔ R shifts to T (shows more cooperativity = sigmoidal curve). AMP (strong activator) binds to the T form and changes it to the R form, or binds to the R form and stabilizes it. - + 0.1 mM AMP AMP activates with 1/10 concentration of ATP. Inhibited 29 Chapter 14 30 Takusagawa’s Note© AMP overcomes the ATP inhibition of PFK - The metabolic flux through glycolysis may vary by 100-fold or more. - But [ATP] varies < 10% between rest and vigorous exertion. Why? Because the inhibition of PFK by ATP is relieved by AMP. - Action of two enzymes, creatine kinase and adenylate kinase, buffer the [ATP]. K = [ATP][AMP]/[ADP]2=0.44 ----- [1] - For adenylate kinase: 2ADP ↔ ATP + AMP Thus, ADP resulting from ATP hydrolysis in muscle equilibrates with ATP and AMP. - In muscle: [ATP] ≈ 4.55 mM, [ADP] ≈ 0.50 mM (~10% of [ATP]), [AMP] ≈ 0.02 mM (~0.5% of [ATP]), i.e., [ATP] >> [AMP]. - For example, 10% decrease in [ATP] causes four-fold (300%) increase of [AMP]. This is proved as follows: Let us assume that the total concentration (AT) of [ATP], [ADP] and [AMP] is 5 mM. Thus, AT = [ATP] + [ADP] + [AMP] ≈ [ATP] + 1/10[ATP] = 1.1[ATP] = 5.0 [ATP] = 5.0/1.1 = 4.55 mM. From equation [1], 2 2 2 2 K eq [ ADP ] 0.44( A T − [ ATP ] − [ AMP ]) 0.44( A T − [ ATP ]) 0.44(5.0 − 4.55) = ≈ = [ AMP] = 4.55 [ ATP] [ ATP] [ ATP] [AMP] = 0.02 mM 10% decrease in [ATP]: [ATP]new = 0.9[ATP]old = 0.9 × 4.55 = 4.09 mM 0.44(5.0 − 4.09) 2 [AMP]new = = 0.089 mM 4.09 Thus [AMP]new/[AMP]old = 0.089/0.020 = 4.45 (more than 4-fold increase). - ~1/10 of [AMP] can overcome the ATP inhibition. Thus, the 4-fold increase [AMP] activates PFK by 40-fold (4 × 10). Therefore, the metabolic signal by decrease of a small amount of ATP is greatly amplified by adenylate kinase activity. - Substrate cycling can increase flux sensitivity - Under physiological condition, Fructose-6-phosphate (F6P) + ATP → fructose-1,6-bisphosphate (FBP) + ADP FBP is hydrolyzed by fructose-1,6-bisphosphatase (FBPase) FBP + H2O → F6P + Pi - Both reactions are exergonic (ΔG < 0). 30 - Takusagawa’s Note© 31 Chapter 14 This ATP wasting cycle can eliminate excess of ATP. [ATP]↓ & J↓ PFK’s activators, AMP and [F2,6P] inhibit FBPase. Much of body heat of many animals is generated through substrate cycle. Substrate cycle is stimulated by thyroid hormones. Summary of PFK regulation J (flux) F6P ATP Pi vf FBPase Substrate Cycle Strong activator PFK Inhibitor vr ADP H2O FBP ATP + AMP Adenylate kinase J A realistic example 1. [ATP] is reduced by 10%. 2. [ADP] is increased. 3. ADP is converted to ATP and AMP by adenylate kinase . 4. AMP is strong activator of PFK, thus relieves the weak ATP inhibition (increase ~10-fold glycolytic flux). 5. AMP inhibits FBPase activity to slow down the substrate cycle. Let us assume that the maximum activities of PFK = 100, FBPase = 10. At low [AMP] (~0.02 mM), activities of PFK = 10% and FBPase = 90% Flux rate J = 100 × 0.1 - 10 × 0.9 = 1 At high [AMP] (~0.09 mM), activities of PFK = 90% and FBPase = 10% Flux rate J = 100 × 0.9 - 10 × 0.1 = 89 Therefore, the flux is increased by ~90-fold in this simple calculation. 31 Takusagawa’s Note© 32 Chapter 14 5. METABOLISM OF HEXOSES OTHER THAN GLUCOSE Other hexoses: Fructose, Galactose, Mannose A. Fructose - Fructose metabolism in muscle differs from that in liver. - In muscle, - Muscle contains enough hexokinase which phosphorylates the fructose. Fructose → fructose-6-phosphate → glycolysis. - In liver, - Liver contains glucokinase (rather than hexokinase) that cannot work on fructose. Thus, fructose must be converted to glycolytic intermediates through a pathway that involves six enzymes. HOCH2 O CH2OH HO Muscle OH ATP Liver ATP HO ADP ADP 2- O3POCH2 O CH2OH HOCH2 O HO 2- CH2OPO3 HO OH OH HO HO H Glycolysis C O six enzyme reactions H C OH 2- CH2OPO3 Glyceraldehyde-3phosphate 32 B. - Takusagawa’s Note© 33 Chapter 14 Galactose Galactose is an epimer of glucose at C4. Enzymes in glycolysis are specific, and do not recognize the galactose conformation. Therefore, galactose must be converted to glucose before entering the glycolysis. Galactose is initially converted to UDP-galactose, and then to UDP-glucose. The UDP-glucose is converted to G6P which enters into glycolysis by skipping hexokinase. UDP-Galactose UDP-Glucose CH2OH CH2OH CH2OH O O O HO HO O UDP OH NAD OH OH OH O UDP OH 2- O PO3 OH HO + NAD CH2OH NADH O O OH + 2- CH2OPO3 O NADH OH O UDP OH HO OH OH G6P - Galactosemia is a genetic disease characterized by an inability to convert galactose to glucose. C. Mannose - Mannose is an epimer of glucose at C2. - Mannose enters the glycolysis after conversion to F6P. CH2OH O OH HO HO 2- ATP OH ADP hexokinase CH2OPO3 O OH HO O3POCH2 O phosphomannose isomerase OH HO F6P Mannose 33 CH2OH HO OH HO -2