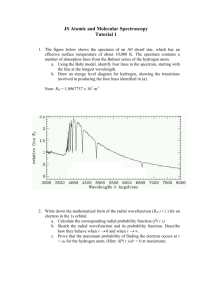
1. In the Geiger-Marsden experiment, α particles are scattered by
advertisement

1. In the Geiger-Marsden experiment, α particles are scattered by gold nuclei. The experimental results indicate that most α particles are A. scattered only at small angles. B. scattered only at large angles. C. absorbed in the target. D. scattered back along the original direction. (1) 2. Which one of the following provides direct evidence for the existence of discrete energy levels in an atom? A. The continuous spectrum of the light emitted by a white-hot metal. B. The line emission spectrum of a gas at low pressure. C. The emission of gamma radiation from radioactive atoms. D. The ionization of gas atoms when bombarded by alpha particles. (1) 3. In an α-particle scattering experiment (Geiger-Marsden experiment), the number n of particles incident per unit time on a detector was determined for different angles of deflection θ. foil incident - particles detector 1 Which of the following graphs best shows the variation with θ of n? A. n –90° B. 0 +90° θ 0 +90° θ 0 +90° θ 0 +90° θ n –90° C. n –90° D. n –90° (1) 2 4. The emission and absorption spectra of different elements provides evidence for the existence of A. isotopes. B. neutrons. C. protons. D. atomic energy levels. (1) 5. Which of the following provides evidence for the existence of atomic energy levels? A. The absorption line spectra of gases B. The existence of isotopes of elements C. Energy release during fission reactions D. The scattering of α -particles by a thin metal film (1) 6. The Geiger-Marsden alpha particle scattering experiment provides evidence for the existence of A. atomic nuclei. B. neutrons. C. protons. D. nuclear energy levels. (1) 3 7. The diagram below shows the path followed by an alpha-particle in the vicinity of the nucleus of a gold atom. alpha-particle nucleus Which of the following is correct for the alpha-particle? A. The force acting on it changes direction. B. The force acting on it is smaller than that acting on the nucleus. C. Its potential energy is constant. D. Its kinetic energy is constant. (1) 4 8. Some of the energy levels of the hydrogen atom are shown below. –––––––––––––– – 0.54 eV –––––––––––––– – 0.85 eV –––––––––––––– – 1.51 eV –––––––––––––– – 3.39 eV –––––––––––––– – 13.6 eV Electrons are excited to the 0.85 eV level. How many different photon frequencies will be observed in the emission spectrum of hydrogen? A. 3 B. 4 C. 5 D. 6 (1) 9. The Bohr model of the hydrogen atom is able to A. predict accurate values for some of the wavelengths in the spectrum of atomic hydrogen. B. account for the detailed structure of the spectral lines in the spectrum of atomic hydrogen. C. explain the relative intensity of the different spectral lines in the spectrum of atomic hydrogen. D. be extended to predict accurately, some of the wavelengths in the spectrum of oxygen. (1) 5 10. The diagram below shows four electron energy levels E3, E2, E1 and E0 in an atom of a gas. E3 E2 E1 increasing energy E0 Which of the following gives the number of lines in the spectrum of the gas that are associated with these energy levels? A. 3 B. 4 C. 5 D. 6 (1) 11. The Bohr model of the hydrogen atom predicts A. the line spectra of multi-electron atoms. B. the wavelengths of the principal lines in the spectrum of atomic hydrogen. C. the wavelengths in the spectrum of molecular hydrogen. D. the relative intensities of the spectral lines of atomic hydrogen. (1) 6 12. The diagram below shows some possible electron transitions between three principal energy levels in the hydrogen atom. Which electron transition is associated with the absorption of a photon of the longest wavelength? 0 eV B D A –1.2 eV C –13.6 eV (1) 13. The diagram below shows three energy levels of a certain atom. 0 T –5.0 eV S –15.0 eV The photon associated with the energy change T has frequency fT and the photon associated with the energy change S has frequency fS The ratio A. 1 . 3 B. 1 . 2 C. 2. D. 3. fS is fT (1) 7 14. The diagram below shows four energy levels in an atom, together with some possible electron transitions. E4 E3 E2 E1 Which one of the following best represents the emission line spectrum produced from these transitions? increasing wavelength A. B. C. D. (1) 8 15. The diagram below shows the three lowest energy levels of an atom of an element. energy n 3 n 2 n 1 Which of the following diagrams best represents the emission spectrum that results from electron transitions between these energy levels? B. A. increasing wavelength C. increasing wavelength D. increasing wavelength increasing wavelength (1) 9