Study sheet for 5-1
advertisement

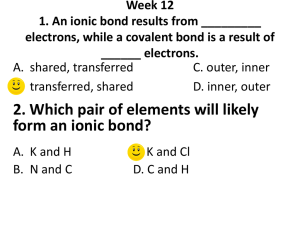
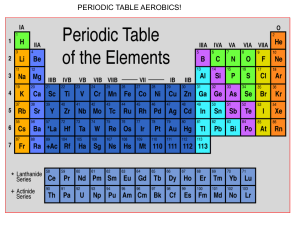
Name ____________________________________________________ This study guide is due at the end of class TODAY for full credit. After that, it is worth only half-credit. Directions: Re-read pp. 158-163 in your textbook in your small group. Answer the following questions—you can work together and help each other, but each person must turn in his/her own study sheet at the end of the hour. 1) Write out the definition of an ion: 2) Look carefully at the electron configuration given for Na and for Na+. What is the difference? 3) Does sodium atom (Na) have the same chemical properties as a sodium ion (Na+)? Why or why not? 4) Define cation: 5) Define anion: 6) Make up a rule that will get you to remember how to say the word “cation” and the positive charge and the word “anion” and the negative charge. Write the rule here (it can be a drawing, if you like): 7) Define electronegativity: 8) Look at the top of page 160. What are they trying to tell you by showing the electron configurations of S, S2-, and Ar ? 9) Look at the Table 5-1. You will have to memorize the element symbols and their charges for groups 1 & 2 for tomorrow (this is your homework---plan on a quiz first thing Tuesday). 10) What is the octet rule? Define it here. 11) Take a look at Table 5-2. Write down three of your observations about the table here: 12) Turn to page 162. Notice that the Copper ion has 2 forms. Write them here: 13) Notice that the name of a simple anion is formed from the element’s name, but it ends in –ide. List three anions here (of your choice) using the correct naming rules (nomenclature): 14) Define ionic compound: 15) What is a binary ionic compound? 16) How do you name simple binary ionic compounds? 17) Write out an example explaining in your own words how to do it: 18) Read p. 163. See how NaCl is balanced with one cation with a positive charge, and one anion with a negative charge. Likewise, ZnS is balanced with one cation with a positive 2 charge, and one anion with a negative 2 charge. So what happens with magnesium nitride? Notice Mg ion has 2 positive charges, but N ion has 3 negative charges. Sooooo they cannot go together like MgS (that compound would not be balanced in charge). They have to go together as Mg3N2. Notice the 3 is a subscript that indicates 3 Mg ions, and the 2 is a subscript that indicates 2 N ions. Now we have a balanced ionic compound. If you have time, practice writing the names of the following compounds: Sodium chloride Lithium chloride Potassium chloride Cesium bromide Magnesium chloride Calcium chloride Beryllium bromide Barium iodide Magnesium oxide Calcium oxide Barium sulfide Magnesium sulfide Do you see any trends here?