Intermolecular Forces: Dipoles, H-Bonds, & Dispersion
advertisement

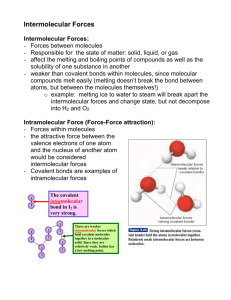
Intermolecular Forces and Strengths How do molecules stick together—even in the worst of times? Why? As you have learned, matter is made up of discrete particles called atoms, which chemically combine to form molecules. Molecules do not exist as independent units: in fact, groups of molecules ‘stick together’ in order to form liquids and solids. The forces that hold groups of molecules together are intermolecular forces. Without intermolecular forces, the world as we know it would not be the same. Model 1: Intramolecular and Intermolecular Forces 1. What is represented inside each box in Model 1? 2. In relation to the box for molecule 1, where do the intramolecular forces exist? 3. Based on the intramolecular forces for molecule 1, draw similar asterisks for the intramolecular forces on the diagram for molecules 2 and 3. 4. In relation to the molecule, where do intramolecular forces tend to occur? 5. Two intermolecular forces exist in Model 1. Where are they positioned relative to the molecules? ©HSPI – The POGIL Project Limited Use by Permission Only – Not for Distribution Intermolecular Forces C1YvM 1 6. State, in everyday language, the difference between intermolecular and intramolecular forces in terms of where they occur on the molecular level. Model 2: Dipole-Dipole Interactions 7. The diagram above represents an arrangement of five molecules. What is the difference between the two ends of each molecule? 8. Molecules with the property you identified in #7 are known as dipoles. Consult with your group and reach a consensus on what you think a dipole is. Write down this definition. 9. Consider the attractive forces shown in Model 2. In terms of the positive and negative ends of the dipoles, where do attractive forces occur? 10. Consider the repulsive forces shown in Model 2. In terms of the positive and negative ends of the dipoles, where do repulsive forces occur? ©HSPI – The POGIL Project Limited Use by Permission Only – Not for Distribution Intermolecular Forces C1YvM 2 11. The forces described in #9 and #10 are called dipole-dipole interactions. How might dipoledipole interactions help many molecules attract each other? Read This! A dipole is most often found in polar covalent molecules, in which the electrons are unevenly shared. This uneven sharing gives one side of the molecule a partially positive charge (δ+) and the other side a partially negative charge (δ–). 12. What causes the dipole in polar covalent molecules? 13. What symbols are used to represent the partial charges at the ends of polar covalent molecules? 14. In the diagram below, a hydrogen iodide molecule has been drawn with its partial positive and partial negative charges. Using the three molecules in the box, draw an attractive force between two HI molecules using a dashed line (- - -) and a repulsive force between two HI molecules using a dotted line (.....) 15. Nonpolar molecules do not form dipoles, because electrons are shared evenly. Would you expect nonpolar molecules to “stick together” more or less effectively than polar molecules? Explain your answer in terms of dipole-dipole interactions. ©HSPI – The POGIL Project Limited Use by Permission Only – Not for Distribution Intermolecular Forces C1YvM 3 Read This! When a hydrogen atom is covalently bonded to nitrogen, oxygen, or fluorine, a very strong dipole is formed. The dipole-dipole interactions that result from these dipoles are known as hydrogen bonding. Hydrogen bonding is an especially strong form of dipole-dipole interaction. 16. Below is a diagram of ammonia. Draw two more ammonia molecules in the box, indicating the partial positive and partial negative ends for each molecule. 17. Draw the attractive forces between the ammonia molecules with a dotted line. Label these forces hydrogen bonds. 18. Indicate and label one covalent bond in the box. 19. Is a hydrogen bond the same as a covalent bond? 20. With your group, explain your answer to #19, including: a. How a covalent bond is formed. b. How a hydrogen bond is formed. c. How the two are similar or different i. Name at least one similarity between a hydrogen bond and a covalent bond. ii. Name at least one difference between a hydrogen bond and a covalent bond. ©HSPI – The POGIL Project Limited Use by Permission Only – Not for Distribution Intermolecular Forces C1YvM 4 Model 3: London Dispersion Forces 21. Bob’s sheep like to wander around the pasture. They could be found anywhere at any moment. How are Bob’s sheep distributed in box A? 22. How are Bob’s sheep distributed in box C? Are the woolly sheep evenly spread in the pasture? 23. Looking at the sheep in box C, identify a ‘woolly’ area and a ‘non-woolly’ area. Label the woolly area W and the non-woolly area NW. 24. Now let’s look at the krypton atoms in boxes B and D. Electrons, like sheep, like to wander around the atom. How many protons and electrons are in the atom in each box? 25. Is each atom electrically neutral? Explain. 26. In box B, are the electrons evenly distributed around the nucleus? Explain. ©HSPI – The POGIL Project Limited Use by Permission Only – Not for Distribution Intermolecular Forces C1YvM 5 27. In box D, are the electrons evenly distributed around the nucleus? Explain. 28. In box D, indicate any area that is crowded with electrons with a “δ-“, and any area where the nucleus is exposed with a “δ+.” 29. Even though the krypton atom in #28 is electrically neutral, why could it be said to have a “momentary” dipole? 30. Based on your answer to #29, what do you think helps neutral atoms and molecules attract each other? Read This! Even though atoms by themselves are electrically neutral, a momentary imbalance of electrons can create a momentary dipole. The dipole-dipole interactions among these momentary dipoles are known as London dispersion forces. These forces, also known as van der Waals forces, help neutral atoms and molecules attract each other. 31. What causes a London dispersion force to occur between two atoms or molecules? 32. How are London dispersion forces similar to dipole-dipole interactions? How are they different? ©HSPI – The POGIL Project Limited Use by Permission Only – Not for Distribution Intermolecular Forces C1YvM 6 Extension Questions 33. What is the difference between the formation of an ion and the formation of a momentary dipole? 34. The difference of electronegativity between hydrogen and bromine is 0.7, and the difference in electronegativity between hydrogen and chlorine is 0.9. Which would exhibit stronger dipole-dipole interactions, HBr or HCl? Justify your answer. 35. Neon has ten electrons, and krypton has eighteen electrons. Which element do you think has a greater chance of forming a momentary dipole? Which element do you think exhibits greater London dispersion forces? ©HSPI – The POGIL Project Limited Use by Permission Only – Not for Distribution Intermolecular Forces C1YvM 7 Teacher Resources Learning Objectives: 1. Student will be able to differentiate between inter- and intramolecular forces. 2. Student will be able to explain the causes for dipole-dipole interactions, hydrogen bonding, and London dispersion forces. Prerequisites: 1. Atomic structure and subatomic charges. 2. Polar and nonpolar covalent bonding. 3. Wave-mechanical model and electron clouds Assessment Questions 1. Which of the following does not exhibit dipole-dipole interactions? (a) H2O (b) CH4 (c) CH3Cl (d) HI 2. Since the electronegativities of the atoms involved in hydrogen bonding are relatively high compared to other atoms in their groups, how might this explain the large dipole forces felt in hydrogen bonding? 3. Explain why nonpolar molecule such as methane (CH4) still experiences attraction among its molecules. Assessment Target Responses 1. B 2. These atoms are capable of pulling the electron far away from the hydrogen atom, thereby creating a large difference in charge from one end of the molecule to the other and hence a large dipole-dipole interaction. 3. Methane is a nonpolar molecule. But, like krypton it is polarizable. Therefore, London dispersion forces exist for methane. Teaching Tips 1. Alternate Models: Many teachers have used inquiry-based lab models in which students measure the boiling points of various substances and then explain their trends while learning the concepts of intermolecular forces. The caveat to these models is that the results of intermolecular forces (e.g., boiling point, vapor pressure, and melting point) are confused with the causes of intermolecular forces. Therefore, the instruction can become muddled. 2. Stop Signs: Have the students call you over to their table, so that you can give them the go-ahead to continue to the next step. 3. Implementation: You may want to ask the group manager (or checker, or whoever is facilitating) to raise their hand periodically to indicate which number their group is on. This activity is fairly time-intensive and requires excellent time management. 4. Common Misconceptions: ©HSPI – The POGIL Project Limited Use by Permission Only – Not for Distribution Intermolecular Forces C1YvM 8 Intermolecular forces, in addition to being caused by bonding, actually exist within the bonds. Only polar species are involved in intermolecular forces. Hydrogen bonds are actual bonds within a molecule, as opposed to intermolecular forces between the separate molecules. Targeted Responses 1. What is represented inside each box in Model 1? Water molecules. 2. In relation to the box for molecule 1, where do the intramolecular forces exist? Inside the box. 3. Based on the intramolecular forces for molecule 1, draw similar asterisks for the intramolecular forces on the diagram for molecules 2 and 3. (Asterisks drawn on rest of the covalent bonds.) 4. In relation to the molecule, where do intramolecular forces tend to occur? Within the molecule. 5. Two intermolecular forces exist in Model 1. Where are they positioned relative to the molecules? They are between the molecules. 6. State, in everyday language, the difference between intermolecular and intramolecular forces in terms of where they occur on the molecular level. Intramolecular forces occur within/inside molecules, while intermolecular forces occur between molecules. 7. The diagram above represents an arrangement of five molecules. What is the difference between the two ends of each molecule? One side is positively charged; the other side is negatively charged. 8. Molecules with the property you identified in #7 are known as dipoles. Consult with your group and reach a consensus on what you think a dipole is. Write down this definition. A dipole is a species with one positively charged end and one negatively charged end. 9. Consider the attractive forces shown in Model 2. In terms of the positive and negative ends of the dipoles, where do attractive forces occur? Attractive forces occur between one positive and one negative end. 10. Consider the repulsive forces shown in Model 2. In terms of the positive and negative ends of the dipoles, where do repulsive forces occur? Repulsive forces occur between two positive ends or between two negative ends. 11. The forces described in #9 and #10 are called dipole-dipole interactions. How might dipole-dipole interactions help many molecules attract each other? Dipoles are held together by the attraction between the oppositely charged ends of the separate molecules for each other. 12. What causes the dipole in polar covalent molecules? Uneven sharing of electrons. 13. How are the partial charges represented for polar covalent molecules? δ- and δ+ 14. In the diagram below, a hydrogen iodide molecule has been drawn with its partial positive and partial negative charges. Using the three molecules in the box, draw an attractive force between two HI molecules using a dashed line (- - -) and a repulsive force between two HI molecules using a dotted line (.....). Repulsive forces should be shown between two ends of the same partial charge. Attractive forces should be drawn between two ends of opposite charge. Answers may vary. ©HSPI – The POGIL Project Limited Use by Permission Only – Not for Distribution Intermolecular Forces C1YvM 9 15. Nonpolar molecules do not form dipoles, because electrons are shared evenly. Would you expect nonpolar molecules to “stick together” more or less effectively than polar molecules? Explain your answer in terms of dipole-dipole interactions. Nonpolar molecules would “stick together” less effectively than polar molecules, because they lack the dipole that would enable them to form positive and negative ends that hold together with dipole-dipole interactions. 16. Below is a diagram of ammonia. Draw two more ammonia molecules in the box, indicating the partial positive and partial negative charges for each molecule. Look for the completed molecules, including the partial charges. 17. Draw the attractive forces between the ammonia molecules with a dotted line. Label these forces hydrogen bonds. The students should label the attractive forces between oppositely charged ends of two different ammonia molecules. They should not label bonds within the molecules themselves. 18. Indicate and label one covalent bond in the box. Students should label a covalent bond within one of the molecules. 19. Is a hydrogen bond the same as a covalent bond? No. 20. With your group, explain your answer to #20, including: a. how a covalent bond is formed. A covalent bond is formed by the sharing of electrons between two atoms. b. how a hydrogen bond is formed. A hydrogen bond is formed by the dipole-dipole interactions between two polar molecules containing hydrogen bonded to nitrogen, oxygen, or fluorine. c. how the two are similar or different. Hydrogen bonding is an intermolecular force that results from uneven electron sharing within the molecule. Covalent bonding is the sharing of the electrons themselves and happens within the molecule. Hydrogen bonding is intermolecular, and covalent bonding is intramolecular. 21. Bob’s sheep like to wander around the pasture. They could be found anywhere at any moment. How are Bob’s sheep distributed in box A? They are distributed evenly throughout the pasture. 22. How are Bob’s sheep distributed in box C? Are the woolly sheep even across the pasture? No, the woolly sheep are not distributed evenly throughout the pasture. 23. Looking at the sheep in box C, identify a ‘woolly’ area and a ‘non-woolly’ area. Label the woolly area W and the non-woolly area NW. The bottom left corner of the square should be labeled with a W. The top right corner of the square should be labeled with an NW. 24. Now let’s look at the krypton atoms in boxes B and D.. Electrons, like sheep, like to wander around the atom. How many protons and electrons are in the atom in these two boxes? There are 18 protons and 18 electrons in each atom. 25. Is each atom electrically neutral? Explain. The atoms in each row are neutral, because they have an equal number of protons and electrons. 26. In box B, are the electrons evenly distributed around the nucleus? Explain evenly, and no area has too many or too few electrons. The electrons are distributed ©HSPI – The POGIL Project Limited Use by Permission Only – Not for Distribution Intermolecular Forces C1YvM 10 27. In box D, are the electrons evenly distributed around the nucleus? Explain. The electrons are distributed unevenly in the bottom row. 28. In box D, indicate any area that is crowded with electrons with a “δ-“, and any area where the nucleus is exposed with a “δ+.”The left side of the square should be labeled partially negative, while the right side should be labeled partially positive. 29. Even though the krypton atom in #29 is electrically neutral, why could it be said to have a momentary dipole? The atom is electrically neutral, because it has an equal number of protons and electrons, but the electrons are all on one side of the atom, so a dipole (uneven distribution of charge) is created. It is temporary, because the electrons continue to move. 30. Based on your answer to #30, what do you think helps neutral atoms and molecules attract each other? An uneven distribution of electrons at a given moment helps neutral atoms and molecules form dipoles that hold the atoms and molecules together. 31. What causes a London dispersion force to occur between two atoms or molecules? A momentary imbalance of electrons throughout the molecule. 32. How are London dispersion forces similar to dipole-dipole interactions? How are they different? They are similar because they both involve the attraction of oppositely charged poles toward each other. They are different, because the dipoles in London dispersion forces are only temporary, whereas dipoledipole interactions involve permanent dipoles in polar covalent molecules. 33. What is the difference between the formation of an ion and the formation of a momentary dipole? An ion is created by the removal or addition of an electron to an electrically neutral atom, thereby giving it a charge. A momentary dipole is created by the temporary imbalance of electrons within an electrically neutral atom (the atom remains neutral). 34. The difference of electronegativity between hydrogen and bromine is 0.7, and the difference in electronegativity between hydrogen and chlorine is 0.9. Which would exhibit stronger dipole-dipole interactions, HBr or HCl? Justify your answer. HCl will exhibit stronger dipole-dipole interactions, because the dipole itself will be stronger—the imbalance of charge within the molecule will be greater. 35. Neon has ten electrons, and krypton has eighteen electrons. Which element do you think has a greater chance of forming a momentary dipole? Which element do you think exhibits greater London dispersion forces? Krypton will exhibit greater London dispersion forces, because it has a greater number of electrons. Therefore, there is a greater probability that its electrons will become imbalanced and create a momentary dipole. ©HSPI – The POGIL Project Limited Use by Permission Only – Not for Distribution Intermolecular Forces C1YvM 11