DETERMINATION OF EQUILIBRIUM CONSTANTS
advertisement

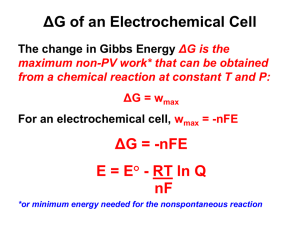
DETERMINATION OF EQUILIBRIUM CONSTANTS USING GALVANIC CELLS PURPOSE The purpose of this experiment is to prepare several electrochemical cells and compare the relative voltages of the half-cells. This will allow us to determine the equilibrium constants for a variety of chemical equilibria. INTRODUCTION The potential (voltage) of a galvanic cell, Figure 1, depends the chemical reactions occurring at the two electrodes (which determines the cell reaction) and on the concentrations of reactants and products. The Nernst equation is an expression that relates the cell potential to the concentrations (and pressures if gases are involved) of reactants and products. In this experiment you will measure the potential and polarity of a few galvanic cells and compare the results with the value calculated from the Nernst equation. You will also use a measured potential to calculate the equilibrium constant for the dissociation of the complex ion Zn(NH3)42+ and the solubility product of AgCl. Some preliminary experiments on cells of known concentration will give you practice in measuring cell potentials and using the Nernst equation. Figure 1. The half-cell solutions are contained in beral pipettes that have had the stems filled with a conducting agar gel. The two half-cells are connected through an external solution of sodium nitrate. To make a measurement, a meter is connected to the two electrodes and the electrodes are placed in the correct half-cell compartment. The potential of a galvanic cell depends on the concentration of solutes and the pressure of gases that participate in the cell reaction. The standard cell potential, E°, is the EMF of a cell in which all solutes or gases that participate in the cell reaction are at unit concentration or pressure. The value of E° and the equation for the cell reaction can be obtained from tables of half-cells potentials such as Table 1. 75 TABLE 1. Standard Electrode Potentials At 25 °C. Half-Cell Reaction E°, V 2+ + 2 e Zn (s) -0.764 2 H+(aq) + 2 e- H2 (g) 0.000 Cu2+(aq) + 2 e- Cu (s) 0.337 Ag+(aq) + e- Ag (s) 0.800 Zn (aq) - The cell consists of two half-cells in contact with an sodium nitrate (NaNO3) solution, which serves as an electrolyte or salt bridge between the half-cell solutions. Medicine droppers contain the half-cell solutions and electrodes. These are inserted into a vial containing the NaNO3 solution. Electrical contact between the ion bridge solution and the half-cell solutions is through the medicine dropper tips (small ends). Because the voltage of a cell depends on the concentrations of the half-cell solutions, plugs of agar salt solutions are placed in the tips of the medicine droppers to keep the NaNO3 solution from physically mixing with the half-cell solutions, which would dilute the half-cell solutions and change the potential of the cell. Because the NaNO3 solution serves only as an ion bridge, leakage of the half-cell solution into the NaNO3 solution does not significantly affect the potential of the cell. The cell voltage, E, which appears in the Nernst equation, must be measured when almost no current is flowing. This zero current potential is known as the electromotive force (EMF). The pH meters we will use to measure the cell potential have very high internal resistance ensuring that very little current flows. If a significant current does flow, then considerable chemical reaction occurs at the electrode surface, and this may cause the electrodes to polarize, i.e., for the concentrations of reactants and products to be different near the electrode surfaces from their concentrations in the bulk of the half-cell solution. Polarization reduces the observed potential for the cell Observation of the polarity of electrodes is as important as the magnitude of the EMF, because the electrical sign of an electrode reveals the direction of the half-reaction occurring at the electrode. At a negative electrode, the half-reaction must supply electrons. If the copper electrode is negative in a copper-copper ion half-cell, then the half-reaction can be described by eqn. 1. Cu(s) Cu2+(aq) + 2 e- (1) The electrons are a product of this reaction and are in excess on the copper electrode. If the copper electrode is positive, then the half-reaction must consume electrons, eqn. 2. Cu2+(aq) + 2 e- Cu(s) (2) Before adding the half-cell reactions, you must reverse one of them and multiply the stoichiometric coefficients by a number that will cause free electrons to cancel out on addition of the half-cell reactions. Before adding the half-cell potentials to obtain the standard cell potential, 76 E°, you must reverse the sign (but leave the magnitude unchanged) of the half-cell potential associated with the half-cell reaction that is reversed. Since the final equation represents the spontaneous reaction only if E° is positive (and the reverse of the spontaneous reaction if E° is negative) it is advisable to reverse the half-cell reaction which will result in a positive sum of half-cell potentials. A sample calculation of E° for this experiment is shown in eqns. 3a-c. + - 2 ( Ag (aq) + e Ag(s) ) Cu(s) Cu2+(aq) + 2 e____________________________________ Cu(s) + 2Ag+(aq) Cu2+(aq) + 2 Ag(s) E°, V +0.800 -0.337 (3a) (3b) +0.463 (3c) The Nernst equation is a relation between the actual cell potential, the standard cell potential, and the concentrations of solutes (or pressures of gases) that participate as reactants or products in the cell reaction. The Nernst equation for any cell is given by eqn. 4a. E = Eo − (4a) RT ln Q nF E is the actual cell potential; E is the standard cell potential; R is the gas law constant; F is Faraday’s constant; T is the Kelvin temperature; n is the moles of electrons transferred in the reaction; and, Q (the so-called reaction quotient) has the same mathematical form as the equilibrium constant expression, i.e. the ratio of the concentrations of products to the concentrations of reactants. Q is numerically equal to Keq only when the reactants and products are in equilibrium (in which case the cell potential, E, will be zero). Thus cell potentials are measured under non-equilibrium conditions. At 25°C, the Nernst equation can be written as: E = Eo − 0.0592 log Q n (4b) Again, the reaction is written as spontaneous only if E is positive, eqn. 5. E = 0.463 − [ ] [ ] Cu 2 + 0.05916 log 2 2 Ag + (5) EXPERIMENTAL PROCEDURE Chemicals: 2 M NaNO3 ; 0.1 M CuSO4; 0.1 M AgNO3; copper wire; silver wire; 0.1 M ZnSO4; a strip of zinc metal; 7 M NH4OH; 0.2 M KCl; Agar Equipment: pH or volt meter with leads and alligator clips; ring stand; clamp; vials; two medicine droppers; steel wool. We will use a voltmeter with the ability to measure millivolts, to determine the potentials of the various cells that you will construct. The meters are equipped with leads and alligator clips that 77 should facilitate attachment to the metal electrodes of your cells. You only need to be sure the meter is turned on and set to read millivolts before you make your measurements. The arrangement of a typical galvanic cell is shown in Figure 1. Clamp a piece of copper wire in each lead from the meter and immerse the unattached ends of the copper wires into a beaker of water. The meter should now read zero. Replace the wire in the negative lead with a strip of zinc metal, and again immerse both electrodes. You should obtain a definite non-zero reading that is, however, not stable or reproducible. Deposits (normally metal oxides) on electrodes or tiny concentrations of ions may affect or even control the potential at an electrode. If the electrodes are not brightly metallic when you receive them, polish them with steel wool. Carefully clean out droppers, beakers and the vials between each experiment, and use a fresh glass wool plug for each half-cell you construct. A. The EMF of the Cell: Cu(s) | Cu2+(aq) (0.1 M) || Ag+(aq) (0.1M) | Ag(s) The above title contains an abbreviated cell description known as a cell diagram. Each single vertical line symbolizes the boundary between two phases, such as an electrode and a half-cell solution in contact with it. The double line symbolizes a salt bridge for ion transport. Clean each of your electrodes to brightness with steel wool followed by rinsing with distilled water. Also be sure that your droppers and vials are rinsed clean. Fill a vial about halfway with 2 M NaNO3 solution and clamp the vial to a ring stand. Your instructor will be providing you with a hot solution of Agar and NaNO3. Using a pipet bulb, draw a small amount of this solution into your dropper and allow it to solidify. Fill the dropper about two-thirds with 0.1 M copper (II) sulfate (CuSO4) solution using the dropper in the stock solution bottle. The solution should not drip from the end and there should not be any air bubbles in the Agar at bottom of the dropper. Place the dropper into the NaNO3 solution in the vial. Insert into the dropper a piece of copper wire. Similarly, prepare a half-cell containing 0.1 M silver nitrate (AgNO3) and a piece of silver wire, and place this Ag | Ag+(aq) half-cell into the NaNO3 solution with the Cu | Cu2+(aq) half-cell. Watch the liquid levels in the droppers and add more CuSO4 or AgNO3 solution if the liquid level in the droppers becomes the same as that of the NaNO3 solution. Attach the meter leads to the copper and silver electrodes in the way that causes a positive potential reading. Record the EMF of the cell and its polarity (i.e., an indication of which electrode is positive). If you do not obtain a stable potential reading, i.e., if the potential changes slowly with time, you probably have an open circuit due to an air bubble in one of the agar solutions. Prepare a new pipet with an agar plug as described above. Record your results in the data section. Use this Nernst equation to calculate the expected EMF of your cell, and compare the calculated value with the measured value. Check whether the direction of the spontaneous reaction is consistent with the observed polarity of the cell. 78 B. EMF of the cell: Zn(s) | Zn2+(aq) (0.1 M) || Cu2+(aq) (0.1 M) | Cu(s) The silver half-cell is to be replaced by the zinc half-cell. Fill another vial about two-thirds full of distilled water in which to store the Ag | Ag+(aq) half-cell during this experiment, but do not actually transfer it until the zinc half-cell is ready to be inserted into the NaNO3 vial. Prepare a zinc half-cell from a dropper, the Agar NaNO3 solution, 0.1 M zinc sulfate (ZnSO4), and a zinc strip. Replace the silver half-cell with the zinc half-cell, and store the silver cell in distilled water. Add appropriate solutions to the half-cells to maintain liquid levels in the droppers slightly above the liquid level in the vial. Measure and record the EMF and the polarity of the zinc-copper cell. Calculate E° for this cell from the data in Table 1. Write the Nernst equation for the cell reaction and calculate the expected value of E. Compare the calculated EMF with the measured EMF. Write the equation for the spontaneous cell reaction, and check whether this is consistent with the observed polarity of the cell. C. EMF of the cell: Zn(s) | Zn2+(aq) (0.1 M) || Ag+(aq) (0.1M) | Ag(s) Exchange the copper and silver half-cells. Measure and record the EMF and the polarity of the zinc-silver cell. Calculate E° and E for this cell. Compare the calculated EMF with the measured EMF. Write the equation for the spontaneous cell reaction, and check whether this is consistent with the observed polarity of the cell. The equation for the spontaneous reaction in this experiment may be obtained by adding the equations for the spontaneous reactions in Parts A and B. In this summation, the copper-copper ion half-cell in the two reactions cancel, and so do their half-cell potentials. This leaves only the zinc-zinc ion and silver-silver ion half-cells, which are the half-cells for Part C. Consequently, the sum of the EMF's from Parts A and B should be equal to the EMF in Part C. Check whether this is consistent with your observations. D. Determination of the Dissociation Constant of the Tetraaminezinc (II) Ion From the EMF of the Cell: Zn(s) | Zn (NH3)42+(aq) (0.05 M) || || Ag+(aq) (0.1M) | Ag(s) In the presence of large ammonia concentrations, zinc ion is converted almost entirely to the tetraamine zinc (II) ion, eqn. 6. Zn2+(aq) + 4 NH3 (aq) Zn(NH3)42+(aq) (6) In this experiment you will form this zinc-ammonia complex ion in one-half of a galvanic cell. You may then calculate the concentration of uncomplexed zinc ion from the measured cell EMF by means of the Nernst equation. This concentration may then be used to calculate the equilibrium constant for dissociation of the complex ion into zinc ion and ammonia. We do not need to worry about the effect of Zn(NH3)42+ on the electrode. Mix in a small beaker twenty drops of 0.1 M zinc sulfate with twenty drops of 7 M ammonia 79 hydroxide. Prepare a half-cell from this solution using a dropper, the Agar solution, the zincammonia solution, and a zinc strip. Construct a cell from this zinc-ammonia half-cell and the silver half-cell. Measure and record the EMF and polarity of the new zinc-silver cell. a. The Zinc Ion Concentration: write the Nernst equation for the cell reaction shown in eqn. 7. Zn(s) + 2 Ag+(aq) Zn2+(aq) + 2 Ag(s) (7) Since you know [Ag+(aq)] and n, have calculated E° for this reaction in Part C, and have measured E, [Zn2+(aq)] is the only unknown quantity in the Nernst equation. Calculate the zinc-ion concentration from the Nernst equation. This is the concentration of uncomplexed zinc ion in equilibrium with ammonia and the tetraaminezinc(II) complex ion b. The Dissociation Constant: the dissociation constant is the equilibrium constant for the reverse of reaction (6) and is given as: K= [Zn ][NH ] [Zn(NH ) ] 2+ (8) 4 3 2+ 3 4 You have just calculated [Zn2+(aq)] from a measurement of EMF. You should have found [Zn2+(aq)] to be quite small, meaning that the concentration of tetraaminezinc (II) complex ion is practically 0.05 M (why not 0.1 M?), i.e., very nearly all of the zinc ion is tied up as Zn(NH3)42+(aq). The initial concentration of ammonia, [NH3(aq)], was 3.5 M and, therefore, [NH3(aq)] after formation of the Zn(NH3)42+(aq) is given 3.5 M - (4x0.05 M) = 3.3 M. Substitute these concentration values into the equilibrium constant expression, and calculate the value of K. E. Determination of the Solubility Product of Silver Chloride, AgCl(s), From the EMF of the Cell: Ag(s) | AgCl(s), Cl-(aq) (0.2 M) || Ag+(aq) (0.1 M) | Ag (s) Plug a dropper with the Agar solution, and fill it about three-fourths full with 0.2 M potassium chloride, KCl, solution. Add one drop of 0.1 M silver nitrate, AgNO3, and mix thoroughly by inverting and re-inverting. This produces a saturated solution of AgCl(s). Insert a silver strip, and form a cell with this half-cell and the silver half-cell whose [Ag+(aq)] is 0.1 M. Measure and record the EMF of this cell and its polarity. The cell thus prepared is known as a concentration cell. Reaction tends to occur in such a way as to make the [Ag+(aq)] in each half-cell the same. Electrons move from the silver electrode in the half-cell with the lower [Ag+(aq)] to Ag+(aq) ions in the half-cell with higher [Ag+(aq)], eqn. 9. Ag+(more concentrated) Ag+ (more dilute) The Nernst equation takes the form of eqn. 10. 80 (9) [ ] [ ] + (10) Ag dilute 0.0592 log E = 0.00V − n Ag + concentrated E° = 0.0 V as no changes in species identities occur; only a change in [Ag+(aq)]. Use the measured value of E and the known [Ag+(aq)] in the more concentrated solution to calculate [Ag+(aq)] in the saturated solution of AgCl(s). The approximate value of [Cl-(aq)] in equilibrium with the AgCl(s) is 0.2 M, assuming that the one drop of 0.1 M AgNO3(aq) did not contain enough [Ag+(aq)] to react with a significant fraction of the Cl-(aq). A better calculation could be made. How? Substitute this [Cl-(aq)] and the calculated [Ag+(aq)] in the solubility product expression, eqn. 11, and calculate a value of Ksp. [ ][ K sp = Ag (+aq ) Cl (−aq ) ] (11) PRELAB Equilibrium Constants using Galvanic Cells. . . . Spring 2012 • • • 1. 2. For short answers, construct clear and concise answers using complete sentences, proper spellings, and correct grammar. For reactions, include all physical state subscripts AND balance the equations. For calculations, show complete problem set-ups, include units, and report answers with the proper number of significant digits. Use data from TABLE 1. Standard Electrode Potentials at 25 °C. Calculate BOTH the standard cell potential (E°cell) and the expected cell potential (Ecell) for the cells listed below. Also, write the overall cell reaction for each of the cells. a. Cu(s) | Cu2+(aq) (0.1 M) || Ag+(aq) (0.1M) | Ag(s) b. Zn(s) | Zn2+(aq) (0.1 M) || Cu2+(aq) (0.1 M) | Cu(s) c. Zn(s) | Zn2+(aq) (0.1 M) || Ag+(aq) (0.1M) | Ag(s) a. Add together the reactions you wrote for cells (a) and (b) above and compare the result to the reaction you wrote for cell (c). Comment on the significance of the comparison. b. Find the sum the answers you found for BOTH the standard cell potential (E°cell) and the expected cell potential (Ecell) for cells (a) and (b) above. Compare the result with the answer you found for part (c). Show the math and comment on the significance of the comparison. Transfer your answers into the appropriate locations in your notebook. 81 Table 2. Sample tables for recording data into your notebook. Part A. The EMF of the Cell: Cu(s) | Cu2+(aq) (0.1 M) || Ag+(aq) (0.1M) | Ag(s) Calculation of E° from the data in Table 1. E° = ________________________ Calculation of E from the Nernst Equation. E= __________________________ Measured Potential (V) ________________ Which Electrode is positive?__________ Comment on the agreement of these values _________________________________ Part B. EMF of the cell: Zn(s) | Zn2+(aq) (0.1 M) || Cu2+(aq) (0.1 M) | Cu(s) Calculation of E° from Table 1. E° = ________________________ Calculation of E from the Nernst Equation. E= __________________________ Measured Potential (V) ________________ Which Electrode is positive?__________ Comment on the agreement of these values: _________________________________ Part C. EMF of the cell: Zn(s) | Zn2+(aq) (0.1 M) || Ag+(aq) (0.1M) | Ag(s) Calculation of E° from Table 1. E° = ________________________ Calculation of E from the Nernst Equation. E = __________________________ Measured Potential (V) ________________ Which Electrode is positive?__________ Compare the calculated potential for this cell to the sum of the calculated potentials from parts A and B. Now compare the measured potential for this cell to the sum the measured potentials from parts A and B. Comment on these comparisons: _____ ____________________________ 82 Part D. Determination of the Dissociation Constant of the Tetraaminezinc (II) Ion From the EMF of the Cell: Zn(s) | Zn(NH3)42+(aq) (0.05 M) || || Ag+(aq) (0.1M) | Ag(s) Measured Potential (V) of the Cell_____________ Corrected Potential (V) of the Cell_____________ (The correction is performed by adding the difference between the calculated and measured values of potential, E, from part C to the measured value in part D.) Calculate [Zn2+(aq)] from the Nernst Equation. [Zn2+(aq)] = _____________________ Calculate the equilibrium constant for the dissociation of Zn(NH3)42+. Calculated Kdiss Accepted Kdiss Kdiss ___________________ Kdiss ___________________ Agreement with tabulated value for K _____ _________ (% error) E. Determination of the Solubility Product of Silver Chloride, AgCl(s), From the EMF of the Cell: Ag(s) | AgCl(s), Cl-(aq) (0.2 M) || Ag+(aq) (0.1 M) | Ag (s) Measured Cell Potential _________________ Calculate [Ag+(aq)] from the Nernst Equation. [Ag+(aq)] ____________________ Calculated Ksp Accepted Ksp Ksp ___________________ Ksp ___________________ Agreement with tabulated value for K _____ _________ (% error) 83 84