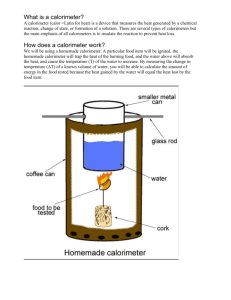
Energy, Heat, and Thermochemistry Worksheet
advertisement

ENERGY is the ability to do work or produce heat 2 forms Kinetic Energy – energy of motion Potential Energy – energy due to the composition or position of an object. (Chemical Potential Energy – energy stored in a substance because of its composition. The potential energy results from the arrangement of the atoms and the strength of the bonds that join them. Stored energy is released when bonds are broken.) Converted into Work Heat – symbol is q; energy in the process of flowing from a warm object to a cooler one units metric calorie (cal) SI kilocalorie called a Calorie with a capital “C” sometimes called nutritional calories 1 Calorie = 1000 calories joule (J) One calorie equals 4.184 joules Conversions: 1. A breakfast of cereal, juice, and milk contains 230 Calories. How much energy in joules will this breakfast supply? 230 Calories 1000 calories 4.184 J 962320 J 1 Calorie 1 calorie 962000 J 2. A fruit and oatmeal bar contains 142 Calories. Convert this energy to calories. 142 Calories 1000 calories = 142 000 calories 1 Calorie 3. An exothermic reaction releases 86.5 kilojoules. (1 kilojoule = 1000 joules) How many kilocalories of energy are released? 86.5 KJ 1000 joules 1 cal 1 KJ 4.184 J 1 Kcal = 20.7 kcal 1000 cal 4. An endothermic process absorbs 256 J, how many kilocalories are absorbed? 256 J 1 cal 1 kcal = 6.37 10-6 kcal 4.184 J 1000 cal Thermochemistry – the study of heat changes that accompany chemical reactions and phase changes Universe System: The specific part of the universe that contains the reaction or process you wish to study. Surroundings: Everything in the universe other than the system. Enthalpy (H) is the heat content of a system. REMEMBER – this is not temperature!! Chemical reactions and phase changes absorb or release heat. To find heat used in a phase change we use ΔHfus and ΔHvap. There is no change in temperature during a phase change. q = m ΔH Enthalpy of fusion (ΔHf) – the energy required to melt a specific amount of substance at its melting point. 1. How much heat is required to melt 5.67 g of iron (II) oxide (FeO) if its enthalpy of fusion is 32.2 kJ/g? q = m ΔHf q = (5.67 g FeO) (32.2 kJ/g) = 183 kJ Enthalpy of vaporization (ΔHv) – the energy required to vaporize a specific amount of a substance at its boiling point. 2. How much heat is required to vaporize 10.0 g of water at its boiling point? ΔHv = 2260 J/g q = m ΔHv q = (10.0 g) (2260 J/g) = 22600 J or 2.26 kJ Remember: If we have a temperature change, we need to use: q = m Cp ΔT If we have a change of state at the boiling or melting point, we need to use: q = m ΔHf or q = m ΔHv 1. How much energy is needed to melt 25.4 g of I2. The ΔHf of I2 is 61.7 J/g. q = m ΔHf q = (25.4 g I2) (61.7 J/g) = 1567.18 J or 1.57 kJ 2. How much energy is needed to heat 10.0 g of ice from –10.0°C to 0.00°C? Cp of ice = 2.06 J/g°C q = m Cp ΔT q = (10.0 g)(2.06 J/g°C)(10.0°C) = 206 J or 0.206 kJ 3. How much energy is needed to melt 45.3 grams of iron at the melting point if the ΔHf of iron is 42.3 kJ/mol? q = m ΔHf Notice: ΔHf is in kJ/mol q=( 45.3 g Fe 1 mole FeO ) (42.3 kJ/mole) = 41.2 kJ 55.8 g Fe Specific Heat – the amount of heat required to raise the temperature of one gram of a substance by one degree Celsius. Each substance has its own specific heat (Cp). For liquid water Cp = 4.184 J/g°C. The heat absorbed or released by a substance during a change in temperature depends on the specific heat, the mass of the substance, and the amount the temperature changes. q = heat absorbed or released Cp m ΔT specific heat mass in grams temperature change Examples: 1. How much heat is absorbed when a 4.68 g piece of metal experiences a temperature change of 182°C? (Cp = .301 J/g°C) q = mCpΔT = (4.68 g)(.301 J/g°C)(182°C) = 256.37 J 256 J 2. The temperature of a sample of water increases from 20.0°C to 46.6°C as it absorbs 5650 J of heat. What is the mass of the sample? ΔT = 46.6°C – 20.0°C = 26.6 °C q = mCpΔT 5650 J = m (4.184 J/g°C)(26.6°C) 5650 J = m (111.2944 J/g) 111.2944 J/g 111.2944 J/g m = 50.766 g 50.8 g Try: 1. How much heat is released to the surroundings when 200 g of water at 96.0 °C cools to 25.0 °C? Answer = 59 400 J