ΔH f
advertisement
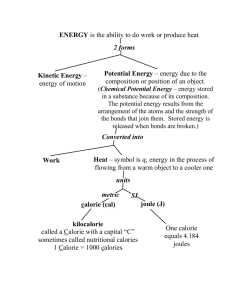
Standard Enthalpy Changes of Reaction 15.1 15.1.1 – Define and apply the terms standard state, standard enthalpy change of formation (ΔHf˚) and standard enthalpy change of combustion (ΔHc˚). Q – What are “standard” conditions? A – 298K (25˚C) around room temp 1.00 x 105 Pa (101.3 kPa) around room pressure standard enthalpy change of formation (ΔHf˚) ΔHf˚= the enthalpy change that occurs when 1 mol of a substance is formed from its elements in their standard states. (see table 11 of IB data booklet) standard enthalpy change of formation (ΔHf˚) Enthaply of formation of any element in its standard state is ZERO !!! ○ For a given state of matter – per standard conditions ○ For a given allotrope – usually the most stable one !!! ○ Superscript may be used to indicate standard conditions Gives a measure of the stability of a substance relative to its elements Can be used to calculate the enthalpy changes of all reactions, hypothetical or real Sample problem 1 Q – the ΔHf˚ of ethanol is given in Table 11 of the IB data booklet. Give the thermo chemical equation which represents the standard enthalpy of formation of ethanol. A – Start with the chemical equation for the formation of ethanol from its component elements in their standard states. _C(graphite) + _H2(g) + _O2(g) _C2H5OH(l) ΔHf˚= -277 kJmol-1 Continue by making the coefficient for ethanol 1 because ΔHf˚ is per mole 2 C(graphite) + 3 H2(g) + ½ O2(g) C2H5OH(l) ΔHf˚= -277 kJmol-1 Sample problem 2 Q – Which of the following does NOT have a standard heat of formation value of zero at 25˚C and 1.00E5 Pa? Cl2(g) I2(s) Br2(g) Na(s) A – Elements in their STANDARD states have a zero value. Bromine is a LIQUID in its standard state, so bromine it its gas state would have a ΔHf˚ not equal to zero (31 Sample problem 3 Q – Which of the following DOES have a standard heat of formation value of zero at 25˚C and 1.00E5 Pa? H(g) Hg(s) C(diamond) Si(s) A – Graphite is more stable (but not harder) than diamond so Si is the only choice in its standard state. [ΔHf˚ C (diamond) = 1.8 kJ/mol] Using ΔHf˚ The following expression is used to predict the standard enthalpy change for an entire reaction. ΔH˚reaction= ΔHf˚products - ΔHf˚reactants Why does this work? ○ Hess’s Law – see worked example in text pp 147148 ΔH˚reaction= ΔHf˚products - ΔHf˚reactants Sample Problem 4 Calculate the enthalpy change for the reaction C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(g) -105 zero 3(-394) 4(-286) kJ/mol ΔH˚reaction= ΔHf˚products - ΔHf˚reactants Sample Problem 4 Calculate the enthalpy change for the reaction C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(g) -105 ΔH˚reaction ΔH˚reaction zero 3(-394) 4(-286) kJ/mol = (3(-394)+4(-286))-(-105) = -2221 kJ/mol ΔH˚reaction= ΔHf˚products - ΔHf˚reactants Sample Problem 5 Calculate the enthalpy change for the reaction C2H5OH (l) + CH3COOH (l) CH3COOC2H5 (l) + H2O(g) -277 kJ/mol -874 -2238 -286 ΔH˚reaction= ΔHf˚products - ΔHf˚reactants Sample Problem 5 Calculate the enthalpy change for the reaction C2H5OH (l) + CH3COOH (l) CH3COOC2H5 (l) + H2O(g) -277 -874 -2238 -286 kJ/mol ΔH˚reaction ΔH˚reaction = [(-2238) + (-286)] – [(-277)+(-874)] = -2524 – (-1151)= -1373 kJ/mol ΔH˚reaction= ΔHf˚products - ΔHf˚reactants Sample Problem 6 Calculate the enthalpy change for the reaction NH4NO3(s) N2O (g) + 2 H2O(g) - 366 + 82 ΔH˚reaction ΔH˚reaction - 285 kJ/mol = [(+82) + 2(-285)] - (-366)] = - 488 – 366 = -122 kJ/mol Practice Problems with Heats of Formation Read Section 15.1 pp 147-149 Do Ex 15.1 # 1-3, 8, 10, 11 ------------------------------------------------------More Practice – Try Talbot – HL Practice Heat of Formation: MC 2, 14, 19, 21, OR 3a