www.njctl.org PSI Biology Membranes & Enzymes Multiple Choice
advertisement

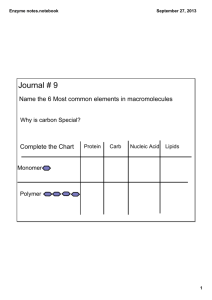
Multiple Choice Review- Membranes & Enzymes 1. Cell membranes are __________________________ and regulate the materials moving into and out of the cell, in order to maintain equilibrium. a. completely permeable b. ionically permeable c. selectively permeable d. protein permeable 2. When we determine the direction and rate of solute movement we must consider the _________________________ both outside and inside of the cell, as well as the magnitude of the concentration gradient. a. The concentration of all solutes added together b. The concentration of water c. The concentration of each solute independently d. The concentration of ions only Use the illustration below to answer questions 3 and 4. 20gNaCl 300ml water 2g NaCl, 20ml water http://www.proprofs.com/quiz-school/story.php?title=practice-regents-2 3. Using the information found within the illustration above, determine the concentration of NaCl outside of the cell and the concentration of NaCl inside of the cell. a. Outside = .67g/ml; inside = 0.1g/ml b. Outside = .067g/ml; inside = 0.1g/ml c. Outside = 66g/ml; inside =1g/ml d. Outside = 132g/ml; inside 10g/ml 4. Based upon the concentrations of NaCl inside and outside of the cell, the outside environment is considered to be ____________________ relative to the inside of the cell. a. hypertonic b. isotonic c. hypotonic d. equatonic www.njctl.org PSI Biology Membranes & Enzymes 5. Solutes and water will diffuse into and out of a cell in order to maintain ________________________. a. equanimity b. a concentration gradient c. equilibrium d. a concentration difference 6. Using the illustration, which of the statements below correctly identifies the movement of the O2 molecules as well as the CO2 molecules? http://www.science-assessments.com/entry/diffusion-cell-water-2 a. b. c. d. O2 will diffuse into the cell while the CO2 will diffuse out of the cell. Both the O2 and the CO2 will diffuse out of the cell Both the O2 and the CO2 will diffuse out of the cell O2 will diffuse out the cell while the CO2 will diffuse into of the cell. 7. A cell will likely shrink or shrivel up when placed into a ______________________ environment. a. Hypotonic b. Hypertonic c. Isotonic d. Neutral tonic 8. During osmosis, diffusion of water molecules will move from a. areas of low solute concentration to areas of high solute concentration b. areas of high solute concentration to areas of low solute concentration c. areas of equal solute concentration to areas of unequal solute concentration d. areas of low water concentration to areas of high water concentration www.njctl.org PSI Biology Membranes & Enzymes 9. Which of the following best describes the characteristics of the fluid mosaic model of cell membranes? a. Fluid because the phospholipids can move and mosaic because the phospholipids form different shapes.. b. Fluid because the membrane has water between the phospholipids and mosaic because the phospholipids differ from each other. c. Fluid because water can diffuse through the membrane and mosaic because different molecule types can also diffuse through. d. Fluid because of the movement of phospholipids and mosaic because of the proteins. 10. What do simple diffusion and facilitated diffusion have in common? a. They both involved the movement of molecules from low concentration to high concentration. b. They both involve the movement of molecules but facilitated diffusion requires energy. c. They both involve the movement of large molecules that require special channels to diffuse. d. They both involve the movement of molecules from high concentration to low concentration. 11. How do carrier proteins and channel proteins differ in their roles within the cell membrane? a. Carrier proteins are integral proteins while channel proteins are peripheral proteins b. Carrier proteins are utilized for both facilitated and active transport while channel proteins function only in passive transport. c. Carrier proteins function in passive transport while channel proteins function in active transport. d. Carrier proteins function in osmosis while channel proteins function in active transport. 12. What is the purpose of active transport if molecules can already move into and out of the cell by diffusion or facilitated transport? a. Some molecules must move down the concentration gradient and energy is required to accomplish this. b. Water molecules need to move even when solutions are isotonic and energy is needed for this. c. Some molecules must move up the concentration gradient and energy is needed to accomplish this. d. Molecules that are too large to pass directly through the phospholipid membrane must use energy to pass through. www.njctl.org PSI Biology Membranes & Enzymes 13. Some molecules are required by the cell for metabolism, however they are larger molecules and have a charge. What is the process most likely used to transport these molecules across the phospholipid bilayer, from higher to lower concentration? a. Passive diffusion b. Osmosis c. Active transport d. Facilitated diffusion Cells are placed in a liquid environment. The concentration of glucose outside of the cells is 30mg/ml. The concentration of glucose inside of the cells is 3g/l. Use this information to answer questions 14 and 15 below: 14. After comparing the concentrations, using like units, which way will the glucose diffuse? a. Out of the cells b. Into the cells c. Net diffusion is equal d. Cannot determine by the information provided 15. Assuming the glucose is the only solute, in which direction will osmosis occur? a. Water will diffuse into the cell. b. Water will diffuse out of the cell. c. Water is already in equilibrium d. The solute will move out of the cell. 16. The illustration below shows a lab set-up to simulate diffusion and osmosis across a semi-permeable membrane. Based upon the information provided, we hypothesize that the sucrose and glucose will diffuse out of the cell. Assuming the bag is equally permeable to sucrose and glucose, what can we predict regarding the rates of diffusion? a. Glucose will diffuse at a higher rate than the sucrose. b. Sucrose will diffuse at a higher rate than the glucose. c. The sucrose and glucose will diffuse at the same rate. d. We cannot compare the rates given the information provided. www.njctl.org PSI Biology Membranes & Enzymes 17. The formation of a membrane has allowed cells to regulate materials coming into and out of the cell. This allows the cell to maintain _____________________. a. an isotonic state b. homeostasis c. pH conditions d. temperature conditions 18. While early cell membranes were primarily composed of phospholipid bilayers alone, cells have evolved to include proteins in their membranes structures. What is the primary function of these proteins? a. They act as “doorways” to allow larger molecules to pass through the cell membrane. b. They act as enzymes to break larger molecules into smaller molecules, to pass through the cell membrane. c. They act only in active transport, to move molecules across the cell membrane. d. They act only in facilitated diffusion as channels to allow molecules to move across the cell membrane. 19. Molarity is one way to measure the concentration of a solution. For a diffusion experiment we are preparing a solution into which we will place a semi-permeable bag. We dissolve 2.00 moles of solute into 1.00 liter of solution. What is the molarity of the solution? a. 0.5M b. 1.0M c. 1.5M d. 2.0M 20. Which of the following characteristics are not associated with catalysts? a. Speed up or slow down reactions b. Speed up reactions c. Remain unchanged d. Less energy need to start a chemical reaction 21. Which of the following best describes the relationship between an enzyme and a substrate? a. Enzymes are specific to many different types of substrates. b. Enzymes are specific to certain substrates. c. Enzymes can be denatured to bind with different substrates. d. Enzymes can only bind with specific proteins. www.njctl.org PSI Biology Membranes & Enzymes 22. The graph below represents the decomposition of a molecule both with and without an enzyme catalyst. The enzyme decreases the energy required for the reaction. How much more energy is required for this reaction to occur, in the absence of the enzyme? a. b. c. d. 80 kJ 60 kJ 20 kJ not enough information 23. Enzymes function optimally in a range of temperature and pH. How might a significant variation from the optimal temperature or pH affect a reaction? a. Changes in pH will increase the rate of reaction; changes in temperature will decrease the rate of reaction b. If the activation site is physically altered, the reaction will not be catalyzed by the enzyme c. The reaction cannot occur at all without the enzyme. d. The reaction will proceed at the same rate because other enzymes can change shape to fit the substrate. 24. The average human body temperature is 37o Celsius. Our immune system may react to a bacterial infection by raising our body temperature, typically referred to as a fever. However, if our temperature increases to 40o C or above for an extended period time, this may cause its own concern. What is the most likely reason for such a concern? a. The optimal temperature for bacterial enzymes are 40oC and above. b. At 40oC and above, human enzymes will be denatured. c. At 40oC and above, bacterial enzymes will be denatured. d. The optimal temperature for viral enzymes are 40oC and above. www.njctl.org PSI Biology Membranes & Enzymes 25. Most biological enzymes have a pH of between ___________. a. 2 and 4 b. 4 and 6 c. 6 and 8 d. 8 and 10 26. Use the data provided in the table below to answer this question: “Normal” values for human blood test for five indicators: Partial pressure of oxygen 75-100 mmHg Partial pressure of carbon dioxide 38-42 mmHg Arterial blood pH 7.38 – 7.42 Oxygen saturation 94 – 100% Bicarbonate 22-28 mEq/L Based upon the data provided, enzymes found in our blood most likely have an optimal pH range, which is ___________________. a. acidic b. basic c. neutral d. acidic or basic Two graphs are seen below. Use the data from these graphs to answer questions 27, 28 and 29 #2 Enzymes #1 #3 #1 Enzymes #2 #3 oC http://academic.brooklyn.cuny.edu/biology/bio 4fv/page/enz_act.htm http://www.theramedix.net/cmsdisplay/therablend.html 27. Describe the environmental conditions under which enzyme #1 functions optimally a. 4oC and pH of approximately 3.8 b. 37oC and pH of approximately 3.8 c. 95oC and pH of approximately 6 d. 95oC and pH of approximately 9 www.njctl.org PSI Biology Membranes & Enzymes 28. Describe the environmental conditions under which enzyme #2 is most likely to denature. a. Temperatures between 30 and 100; pH of 9 b. Temperatures between 0 and 30; pH of 5 c. Temperatures between 30 and 100; pH of 6 d. Temperatures between 0 and 30; pH of 6 29. Which of the three enzymes, (1, 2 or 3 above) is most likely to be found within the human stomach? a. Not enough information b. Enzyme #1 c. Enzyme #2 d. Enzyme #3 30. Coenzymes are: a. Organic molecules that replace enzymes b. Organic or inorganic molecules that are a substitute substrate c. Inorganic molecules that replace enzyme d. Organic molecules that bind at the active site 31. Increasing temperature and the presence of a catalyst may produce similar results in reactions. Why is this? a. The both may produce collisions of greater frequency and energy. b. The both may increase the concentration of an enzyme. c. They both may produce collisions of lesser frequency and energy. d. They both may increase the concentration of a reactant. 32. Which of the following is not true regarding the active site of an enzyme? a. It is specific to a substrate b. It may require a cofactor c. It can be denatured d. It can be utilized only once 33. Which of the following best describes how a competitive inhibitor may affect the enzyme substrate complex? a. A competitive inhibitor binds to the active site of an enzyme. b. A competitive inhibitor binds to an area away from the active site on an enzyme. c. A competitive inhibitor is a substrate that binds to the active site preventing the true substrate from binding. d. A competitive inhibitor is an enzyme that binds to a substrate but does not cause the intended reaction to occur. www.njctl.org PSI Biology Membranes & Enzymes 34. The image below represents a type of inhibition. Which type of inhibition is seen in this illustration? http://www.uic.edu/classes/bios/bios100/ mike/spring2003/ a. b. c. d. Noncompetitive inhibition Competitive inhibition Accidental inhibition Irreversible inhibition 35. Increasing the concentration of the substrate will most likely a. Inhibit the reaction b. Inhibit the enzyme’s function c. Increase the enzyme concentration d. Increase the rate of the reaction 36. What is one way in which vitamins may play a role in enzyme functions? a. Vitamins may play a role as coenzymes b. Vitamins may play a role as an enzyme c. Vitamins typically decrease enzyme activity d. Vitamins prevent infections allowing for enzyme reactions. 37. In allosteric regulation, which of the following may occur? a. Reactions can be either inhibited or activated with this type of regulation. b. Reactions can be inhibited only. c. Reactions can be activated only d. Reactions are not altered, only the enzyme. www.njctl.org PSI Biology Membranes & Enzymes 38. The illustration below represents a process where a product is regulated. In this way, it prevents an overabundance of a product from being produced. What is this process shown in the illustration below? http://cnx.org/content/m44429/latest/ a. b. c. d. Allosteric activation Feedback activation Allosteric denaturation Feedback inhibition 39. If you have a solution containing of 10 grams of NaCl in 200 mL of water, what is the molarity of your solution? a. 0.43M NaCl b. 0.05M NaCl c. 20M NaCl d. 0.855 NaCl 40. Epinephrine (a human hormone) has the formula C9H13O3N. Suppose a molarity of 10M C9H13O3N was considered a “normal” concentration. Upon measurement, the molarity is currently 5M C9H13O3N. How much more epinephrine must be produced to reach normal levels? a. Half as much b. Twice as much c. Three times as much d. Cannot answer with information provided. www.njctl.org PSI Biology Membranes & Enzymes Answer Key Question # Correct response Question # correct response 1 C 21 B 2 C 22 C 3 B 23 B 4 A 24 B 5 C 25 C 6 A 26 B 7 B 27 B 8 A 28 D 9 D 29 B 10 D 30 D 11 B 31 A 12 C 32 D 13 D 33 A 14 C 34 B 15 C 35 D 16 A 36 A 17 B 37 A 18 A 38 D 19 D 39 D 20 A 40 B www.njctl.org PSI Biology Membranes & Enzymes