Point Groups
advertisement

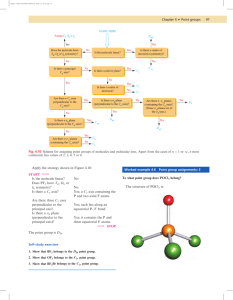
Molecular Spectroscopy January 29, 2008 Molecular Groups, Projection Diagrams, and PointGroups Element Operation Example E, the identity Does nothing All objects posses E Cn, n-fold rotation •Rotate object by 2π/n about the n-fold axis. •The axis with the highest n is called the principle axis. •For n>2 the sense is important…Cn+ - clockwise rotation…Cn--counterclockwise rotation •H2O posses a C2 axis •NH3 posses a C3 axis •Benzene posses a C2, C3, C6, and two sets of three C2 axes perpendicular to the C6 axis σ, reflection plane Reflects through the plane Three types σv, vertical plane Plane containing the principle axis σh, horizontal plane Plane perpendicular to the principle axis •Benzene σd, dihedral plane Vertical plane which bisects two C2 axes perpendicular to the principal axis i, inversion center Sends each atom through the origin along a straight line so that x→-x, y→-y, and z→-z •H2 •CO2 •Benzene •SF6 Rotate object by 2π/n, then reflects through plane perpendicular to the axis of rotation •CH4 Sn, n-fold improper rotation axis •H2O •Benzene Let’s take the ECLIPSED conformation of ethane • One three-fold axis coincident with the C-C bond •Three two-fold axes perpendicular to the C-C bond and intersecting its midpoint •Three reflection planes, each containing the C-C bond and a pair of C-H bonds •One reflection plane perpendicular to the C-C bond and bisecting it •NO CENTER OF INVERSION IS PRESENT •One three-fold improper axis coincident with the C-C bond Let’s take the STAGGERED conformation of ethane • One three fold axis coincident with the C-C bond •Three two-fold axes perpendicular to the C-C bond and intersecting its midpoint •Three reflection planes, each containing the C-C bond and a pair of C-H bond •No reflection plane perpendicular to the C-C bond…we lost it! •One point of inversion at the midpoint of the C-C bond…we gained it! •One six-fold improper axis coincident with the C-C bond…changed its order! Stereographic Projections Let’s consider the symmetry elements of the C2v point group and the resulting symmetry operations Still considering the symmetry elements of the C2v point group and the resulting symmetry operations Let’s compare our results with the C2v character table… C2v E C2 σv σv' E E C2 σv σv' C2 C2 E σv' σv σv σv σv' E C2 σv' σv' σv C2 E Lets try this with another point group and build the character table… C2h E C2 σh i E C2 σh i ⎧ ⎪ ⎪ ⎪ ⎪ ⎪ ⎪ →⎨ ⎪ ⎪ ⎪ ⎪ ⎪ ⎪⎩ → → → → This gives us the following for the Character Table… C2h E E C2 σh i i C2 σh σh C2 i E Complete the rest as we have done and hand it in on Tuesday Point Groups • Symmetry operations may be organized into groups that obey the four properties of mathematical groups…namely… 1. 2. 3. 4. • There exists an identity operator that commutes with all other members The product of any two members must also be a member Multiplication is associative The existence of an inverse for each member It will be possible to assign ANY molecule to one of these so-called Point Groups ¾ So named because the symmetry operation leaves one point unchanged Point Groups • Point Group C1 ¾Trivial group with contains all molecules having no symmetry ¾There is no center of inversion, plane of reflection, improper axis of rotation, etc. ¾The molecule HNClF (below) is such a molecule Point Groups • Point Group Cs ¾Only molecules which have to them a plane of reflection as a symmetry element ¾Formyl Chloride belongs to this point group Point Groups • Point Group Ci ¾Molecules whose sole symmetry element is an inversion center ¾As an example take the rotamer of 1,2-dichloro-1,2-diflouroethane in which the fluorine atoms are off-set to one another ¾The only operations generated by this inversion are i and i2=E…this is a group of order 2 and is designated Ci Important to note that i is the same as an S2 axis Point Groups • Point Group Cn ¾Molecules with only an n-fold axis of rotation ¾Boric acid with the hydrogen atoms lying above the BO3 plane is an example Point Groups • Point Group Cnv ¾Molecules with an n-fold Cn axis of rotation and n vertical mirror planes which are colinear with the Cn axis ¾Water belongs to such a point group…the C2V Note that if you find a Cn axis and one σv plane then you are gauranteed to find n σv planes Point Groups • Point Group Cnh ¾This point group is generated by a Cn axis of rotation AND a horizontal mirror plane, σh, which is perpendicular to the Cn axis ¾Trans-butadiene (trans-1,3-butene) is an example of the C2h point group Point Groups • Point Group Dn ¾This point group is generated by a Cn axis of rotation and a C2 axis which is perpendicular to the Cn axis…no mirror planes ¾Tris(ethylenediamine)cobalt(III) posses idealized D3 symmetry Build this molecule and convince yourself that this in indeed the case!! Point Groups • Point Group Dnd ¾This point group is generated by a Cn axis of rotation and a C2 axis which is perpendicular to the Cn axis and a dihedral mirror plane, σd ¾The dihedral plane is colinear with the principal axis and bisects the perpendicular C2 axis ¾Crystals of Cs2CuCl4 have a squashed tetrahedral D2d structure Point Goups • Point Group Dnh ¾This point group is generated by a Cn axis of rotation and n C2 axis which is perpendicular to the Cn axis and a horizontal mirror plane which is by definition perpendicular to the principal axis of rotation ¾Benzene is the classic example, possesing D6h symmetry Point Goups • Point Groups Sn ¾These point groups are generated by an Sn axis. ¾For example, the spirononane…tetraflourospirononane belongs to the S4 point group and only elements generated by S4 ¾S4 ¾S42 = C2 ¾S43 ¾S44 = E ¾The S2 point group is just Ci since S2 = i ¾When n is odd the point groups are just the same as the Cnh point groups and so are designated as such ¾As a direct result, only S4, S6, and S8, …have a separate existence Special Point Goups • Tetrahedral molecules belong to the point group Td •Octahedral molecules belong to the point group Oh •Molecules with icosahedral (20 triangular faces) or dodecahedral (12 pentagonal faces) belong to the point group Ih • Atoms…which have spherical symmetry, belong to the point group Kh • The point group Th, which may be obtained by adding to Td as set of σh planes, which contain pairs of C2 axis…as opposed to the σd planes which contain one C2 axis and bisect another pair, giving Td)