Pre-Lab for: Synthesis and Acetylation of Ferrocene
advertisement

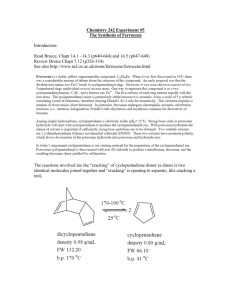
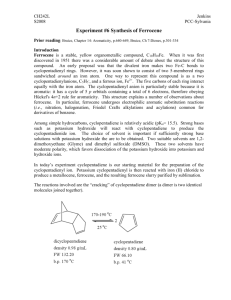
Pre-Lab for: Synthesis and Acetylation of Ferrocene Name: This is our first 2-part experiment. You can approach this in two ways: you can copy both data tables into the introduction, and follow it by both experimentals; or you can copy the ferrocene data table in first, write its experimental, then the data table and experimental for the acetylation after. Either approach is acceptable. 2 KOH 2 Fe FeCl2 cyclopentadiene ferrocene 1. Complete the table with all information useful to the reaction. Show calculations beneath the table, and use SF and units. Reminder: you don't need to fill in the ENTIRE table. Compound FW (g/mol) potassium hydroxide iron (II) chloride tetrahydrate, FeCl2·4H2O cyclopentadiene ferrocene 2. Calculate the theoretical yield of ferrocene. g vol mmol Other (d, M, bp) O Fe O Fe O O H3PO4 acetylferrocene ferrocene 3. Complete the table with all information useful to the reaction. Show calculations beneath the table, and use SF and units. Compound FW (g/mol) g ferrocene acetic anhydride 85% phosphoric acid acetylferrocene 4. Calculate the theoretical yield of acetylferrocene. vol mmol Other (d, M, bp) Synthesis and Acetylation of Ferrocene Ferrocene was first prepared in 1951, and consists of a ferrous ion (Fe2+) sandwiched between two cyclopentadienyl anions. The structure of ferrocene initiated an explosion of interest in metal-carbon bonds and initiated the development of organometallic chemistry. In this experiment we will synthesize ferrocene and then acetylate it through an electrophilic aromatic substitution reaction. The synthesis of ferrocene will involve reaction of cyclopentadiene with KOH to form the aromatic cyclopentadienyl anion, then complexation of two of these anions with Fe2+ to form ferrocene. Ferrocene is airstable, but the synthetic intermediates (cyclopentadiene and Fe2+) are susceptible to oxidation and decomposition by oxygen and water. Therefore during the reaction we will use oven-dried glassware and a balloon of nitrogen to exclude oxygen and water. 2 2 KOH FeCl2 2 Fe2+ Fe = cyclopentadiene ferrocene Cyclopentadiene is a reactive diene that readily undergoes a Diels Alder reaction with itself to form dicyclopentadiene. This dimer is the main form present in the reagent bottle. It can be converted back into monomeric cyclopentadiene by careful fractional distillation (“cracking”) where a retro Diels Alder mechanism occurs. 2 cracking dimerization 2 dicyclopentadiene The acetylation of ferrocene will involve reaction of ferrocene with acetic anhydride and acid through an electrophilic aromatic substitution, as shown below. O Fe O O Fe O H3PO4 ferrocene acetylferrocene Lab Overview: Lab One: Synthesize ferrocene using inert atmosphere conditions. Collect the solid product and purify it through sublimation (Parts A+B). Lab Two: Record the yield of ferrocene. One student should take a 60 MHz 1H NMR of ferrocene and distribute. Acetylate the ferrocene with a partner (Part C) and experiment with TLC’s to find the appropriate solvent system for column chromatography. Lab Three: Purify the acetylferrocene through column chromatography (Part D) and obtain its NMR (try 60 MHz). Experimental: Cracking of Dicyclopentadiene (to be begun the morning preceding lab): “Crack” 15 mL of dicyclopentadiene using a heating mantle and fractional distillation column, with slow collection of the material that distills between 42-45 °C (Cyclopentadiene’s bp= 42.5 oC and dicyclopentadiene’s bp=170 oC). Submerge the receiving flask in an ice bath. Part A: Synthesis of Ferrocene Analytical masses are not necessary for any reagents in Part A. All liquid reagents will be added via syringe, but the needles do not need to be oven dried as small amounts of water/oxygen will not likely ruin the reaction. Cool and flush an oven-dried 25 mL round bottomed flask equipped with stir bar under a balloon of nitrogen gas. When cool, quickly add finely ground KOH (0.75 g, very hygroscopic) to the flask along with 1,2dimethoxyethane (1.25 mL). Again flush the flask with N2 gas and stir to dissolve the KOH. Cool and flush an oven-dried centrifuge tube (16x100mm) equipped with a stir bar and fitted with a rubber septum under a balloon of nitrogen gas (can use the same balloon from the KOH flask). When cool, add iron (II) chloride tetrahydrate (FeCl2·4H2O, fine powder, 0.35 g) along with dimethyl sulfoxide (DMSO, 1.5 mL). Wear gloves and use caution during this addition, as DMSO is readily absorbed through the skin. Stir the solution to dissolve the iron reagent (some careful warming may be necessary to fully dissolve). Transfer the N2 balloon to the round bottomed flask, and add recently distilled cyclopentadiene (0.30 mL, precisely measured). Stir the solution for 5 minutes. The solution may turn a deep burgundy, green or black color as potassium cyclopentadienide is formed. Add the iron/DMSO solution to the round bottomed flask in six 0.25 mL portions over a period of 10 minutes, (one addition roughly every 1.5 minutes), allowing the solution to stir in between additions. After the final addition, rinse the test tube with a fresh 0.25 mL portion of DMSO and add the rinsings to the round bottomed flask. After the iron/DMSO addition is complete, stir the reaction an additional 10 minutes. While wearing gloves, pour the dark slurry and stir bar into a beaker containing ice (5 g) and 6 M HCl (5 mL), and stir until the ice has melted. Collect the orange ferrocene solid via suction filtration using a Hirsch funnel, and wash with several portions of water to remove any blue color (from Fe(C5H5)2+). Dry the ferrocene slightly by squeezing the filter paper from the Hirsch funnel containing the crude ferrocene between sheets of fresh filter paper to press out any excess water. Part B: Sublimation of Ferrocene Spread the crude ferrocene in a thin layer onto the bottom of a petri dish (remove filter paper!), and cap with a second petri dish. Place a beaker containing an ice-water slurry atop the second petri dish. Place the entire setup atop a wire mesh and hotplate in the fume hood, with the hotplate set to mild-medium heat, to avoid charring the ferrocene. If blackening of the ferrocene is seen, reduce the heat. If a small amount of condensation is seen on the top petri dish at the very beginning of the sublimation, wipe it off with a paper towel. However, do not use this method after the beginning stages of the sublimation, or you will breathe toxic ferrocene vapors. ice/water sublimed ferrocene will collect here crude ferrocene wire mesh hotplate on "3-4" The sublimation is finished when the crystals are no longer on the bottom petri dish (or they are all charred), and have all collected on the top or sides of the petri dish. Once finished, remove the petri dishes from the hotplate, while still held together, and allow them to cool. Do not remove the dishes from one another while hot in order to avoid inhalation hazards. If time is an issue, place the two petri dishes (held together) in your locker until the next lab session. Wear gloves whenever handling ferrocene (or when cleaning glassware). Obtain a yield of the sublimed ferrocene. [At least one student should take a 60 MHz 1H NMR of ferrocene and distribute the spectrum to everyone.] Clean-up Notes for Part A+B: • The test tube with residual Fe2+/DMSO should be rinsed into the waste container with water. • If any glassware has yellow-brown stains that don’t seem to be easily cleaned, don’t waste tons of acetone on it. Set this iron-stained glassware aside for our stockroom staff. • Ferrocene is toxic (especially to aquatic animals). Therefore we will collect the filter paper from the suction filtration that contains residual ferrocene. • Before leaving, wipe down the benchtop with a sponge, taking care to remove all specks of orange ferrocene. Part C: Acetylation of Ferrocene Perform this procedure in partners, and wear gloves whenever handling ferrocene or acetylferrocene. Add purified ferrocene (0.093 g) to a 10 mL round bottomed flask containing a stir bar, along with acetic anhydride (0.35 mL). Add concentrated phosphoric acid (85%, 0.10 mL) drop wise via plastic graduated pipette (exothermic addition) while swirling. Attach a CaCl2 drying tube to the round bottomed flask and heat the solution in a 50 oC warm water bath for 10 minutes. Pour the reaction mixture into a 100 mL beaker containing crushed ice (1.5 g), and rinse the reaction flask with water (1.0 mL). Add an NaOH solution (10%, 2.5 mL) to the beaker to destroy the acetic anhydride and neutralize the acid. Test the pH of the solution, and add drops of aqueous saturated NaHCO3 until the solution is near pH 8. Cool the mixture in an ice bath, then collect the solid product by suction filtration using a Hirsch funnel. Wash the solid with a few mL of distilled water and squeeze between sheets of fresh filter paper to remove excess water. Run a TLC of your product alongside the starting material ferrocene, using an eluent composed of hexane and ethyl acetate. As a class, together experiment with different ratios of hexane to ethyl acetate in order to determine the best solvent system for future column chromatography. The ideal column conditions are for the compound of interest (acetylferrocene) to have an Rf near 0.3, and to be separated from any other spots by at least 0.2 (if possible). The spots may be faintly visible after the TLC is run, but can also be visualized with UV. Clean-up Notes for Part C: • Collect the filter paper from the suction filtration into a waste jar, as they contain toxic ferrocene and acetylferrocene. Part D: Column Chromatography Purify the acetylferrocene using column chromatography and the solvent system previously determined to be optimal by the class. Pack the silica gel column to only 4” high, and dissolve the crude product in the smallest amount of CH2Cl2 possible (will be much less than on the video). Collect the fractions into small test tubes. When ferrocene has eluted off the column, increase the solvent polarity to more rapidly elute the acetylferrocene. When all colored components have eluted, combine all fractions containing apparently pure acetylferrocene into a round bottomed flask (rinse test tubes into the flask with a small amount of acetone). Remove the solvent using the rotary evaporator. Obtain a yield, and 60 MHz 1H NMR spectrum of the acetylferrocene. Run a final TLC to ascertain purity. All fractions containing apparently pure ferrocene will be combined by all classmates, and solvent removed by rotary evaporator to be used in future classes. Clean-up Notes for Part D: • Rinse all column test tubes with acetone and clean with soap and water. • Dry the column by pushing air through it, and collect the dry silica in a solid waste jar. Then clean the column with soap and water. Notebook Comments: • Even though you did not personally do the “cracking” part of the procedure, it is important to record this in your procedure because it was essential to the reaction’s success. • This is a 2-part experiment: to make ferrocene, then to make acetylferrocene. Therefore there should be two percent yields.