Review Questions
advertisement

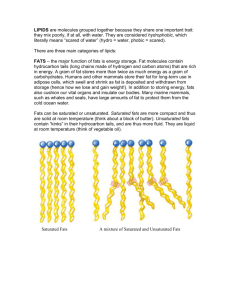
Review Questions Lipids 1. Describe lipids. Lipids are a class of organic compounds that are insoluble in water. Simply put, lipids are non-polar and so cannot mix with water. However, lipids are soluble in other lipids and some organic solvents like chloroform, benzene, and ether. 2. What are the major functions of lipids? Lipids are a long-term storage compound for chemical energy. High in calories, lipids are an energy concentrate, perfect for keeping mobile animals streamlined. Plant oils are mostly found in the seed. The parent plant stores, not only a good supply of starch for its developing embryo, but also some lipid to keep it well fed during germination and early growth. Lipids are poor conductors of heat, making them fantastic insulators. Most animals have a layer of subcutaneous fat but marine mammals have inches of insulating blubber that keep them warm in cold ocean waters. Lipids cushion organs. Our underlying muscles are protected from trauma by a fatty hypodermis. We have fatty pads in our knees. Our kidneys are surrounded by a layer of fat. Fat covers our hearts, internal viscera, spinal cord and eyeballs. All this fat protects these vital organs from the bumps and bruises of life. Hooray for fat! Lipids are waterproofing. Waterfowl stay dry and buoyant by coating their feathers in oil. Waxing your car protects it from the corrosive power of water. 3. What kinds of lipids are there? Lipids are a diverse group of compounds. The most familiar lipids are the neutral fats: animal fats and plant oils. Other lipids include steroids, phospholipids (a lipid of universal importance in every cell), waxes, terpenes, and prostaglandins. 4. Describe the monomer and polymer of the neutral fats. The monomers of the neutral fats are glycerol and fatty acid. Glycerol is a 3-carbon alcohol that serves as the backbone of a neutral fat molecule. Fatty acids are long chains of carbon and hydrogen. Fatty acids are the energy store of the neutral fats. They are hydrocarbons, meaning lots and lots of high energy carbon-to-hydrogen bonds. At the end of the fatty acid is an ester that will bond to the glycerol. The neutral fat polymer is called a triglyceride. Dehydration synthesis bonds 3 fatty acid chains to the glycerol molecule and removes 3 water molecules. Just like the other classes of organic compounds, triglycerides are reduced to glycerols and fatty acids again through hydrolysis. 5. What is the difference between fats and oils? Fats are from animal sources, solid at room temperature, and are saturated. Oils usually come from plants, stay liquid at room temperature, and are unsaturated. 6. Explain the difference between saturated and unsaturated fats. The difference between saturated and unsaturated fats is due to two types of carbon bonding and the resultant change in the number of hydrogen atoms in the fatty acid chains. Saturated fats have fatty acid chains where each carbon is bonded to the carbons on either side by single covalent bonds. The fatty acid is called saturated because every bonding spot where a hydrogen can fit, a hydrogen is there. We can’t jam in any more hydrogen atoms into the compound, so it is “saturated“ with hydrogen. The single covalent bonds in the fatty acid chains make the triglyceride molecule stiff and compact. The result is a fat that has a solid consistency at room temperature. Saturated fats are solids. The fatty acid chains in unsaturated fats have the occasional double bond between the carbons. Since this arrangement leaves some spaces open for additional hydrogens, the fatty acid still has room for more hydrogens and is therefore unsaturated. Each carbon to carbon double bond forms a kink in the chain and acts like a ball and socket joint making the fatty acid mobile. The more double bonds there are, the more joints, and thus more motion. This makes unsaturated fats liquid at room temperature. 7. What is the difference between a monounsaturated and a polyunsaturated fat? When there is only one double bond in each fatty acid, the fat is monounsaturated. Olive oil is mostly monounsaturated fat. If the fatty acids have more than one double bond, it is polyunsaturated. Canola oil is mostly polyunsaturated fat. 8. Why are unsaturated fats considered healthier? It has to do with the calorie content. The carbon-hydrogen covalent bond is packed with energy. The more of these bonds in a molecule, the more calories in the molecule has. Since saturated fats have a maximum number of carbon-hydrogen bonds, they have the most calories. In our calorie-conscious times, saturated fats are super energy rich. So when choosing a lower calorie fat, unsaturated is a healthier choice. 9. What is “partially hydrogenated vegetable oil”? Plant oils are liquid at room temperature because they are unsaturated. But what if you needed a plant oil to turn solid? Well, you could stick it in the refrigerator or mess with the chemistry. Ever eat “natural” peanut butter? If you have, you know that you have to stir it before you use it because of the layer of oil that collects on the surface. Most peanut butter sold in stores doesn’t need to be stirred because the peanut oil has been transformed into a solid through some modern chemistry. By breaking the carbon-to-carbon double bonds and adding hydrogen atoms to the fatty acid chains, hydrogenation transforms a liquid unsaturated fat into a solid saturated fat. Margarines, salad dressings, and a host of other food products have been hydrogenated. 10. Compare the energy content of fats, carbohydrates, and proteins. Fats have the greatest calorie content at a whopping 9 Calories per gram. Carbohydrates and proteins only have 4 Calories per gram a piece. 11. Describe phospholipids. A phospholipid is similar in structure to a triglyceride. There is a glycerol backbone, two fatty acid chains bonded to the glycerol. But instead of a third fatty acid chain in the mix, there is a phosphate group. Now phosphate is a polyatomic ion: basically a negatively charged molecule made of a phosphorus atom surrounded by oxygen atoms. A phospholipid is unique among lipids. One part of the molecule is like a typical fat, non-polar. The other part is charged and will bond to polar molecules like water. So the phosphate group end is called the hydrophilic region (“water loving”) and the fat end is called the hydrophobic region (“water fearing”). The dual nature of the molecule creates a useful structure when placed in water; the phospholipid bilayer. One layer of phospholipids is oriented 180° opposite to the other with the hydrophilic ends pointed outwards toward the water and the fatty acid chains pointing inward. The fatty acid chains of the phospholipids are unsaturated making the bilayer a liquid at room temperature. The bilayer makes an excellent semi-permeable membrane by letting some substances pass through and restricting the passage of other types of molecules. This is great for maintaining the internal complexity of a cell. The outer boundaries plus any internal membranes of all cells are phospholipid bilayers. 12. Describe steroids. Steroids are lipids. Steroids have a structure that is very different from triglycerides or phospholipids. The core of every steroid is four fused carbon rings. One steroid is different from each other is the side chains that arise from this central core. Most hormones are proteins but there are steroid hormones, like sex hormones (testosterone, estrogen, progesterone), and glucocorticoid hormones. Secreted by the adrenal glands, glucocorticoids such as cortisol and aldosterone help regulate metabolic activity, (like the oxidation of glucose) reduce inflammation, and maintain the water balance of the body. One of the most interesting steroids is cholesterol. We know that too much cholesterol leads to artery clogging that cause heart attacks and strokes. But cholesterol is a vital molecule. Cholesterol is the precursor for all other steroids. Every steroid is built from a cholesterol molecule. Cholesterol is a principal component in animal cell membranes. It stabilizes the membrane and strengthens its impermeability to other biological molecules. Cholesterol is an important part of bile salts. Bile is a yellow-greenish fluid secreted by the liver and transported via ducts to the small intestine. The function of bile is to emulsify fats. Emulsification is the reduction in size of fat droplets. Emulsification makes it easier for lipases (enzymes that break down fat) to do their job. Cholesterol is also the precursor of vitamin D. Exposure of sunlight on the skin triggers the conversion. Vitamin D is required by the body for calcium absorption. Sometimes children who don’t get enough sunlight can develop rickets (a disease characterized by soft bones and bowed legs).