Formula of a Hydrate Lab: MgSO4 x H2O Calculation
advertisement
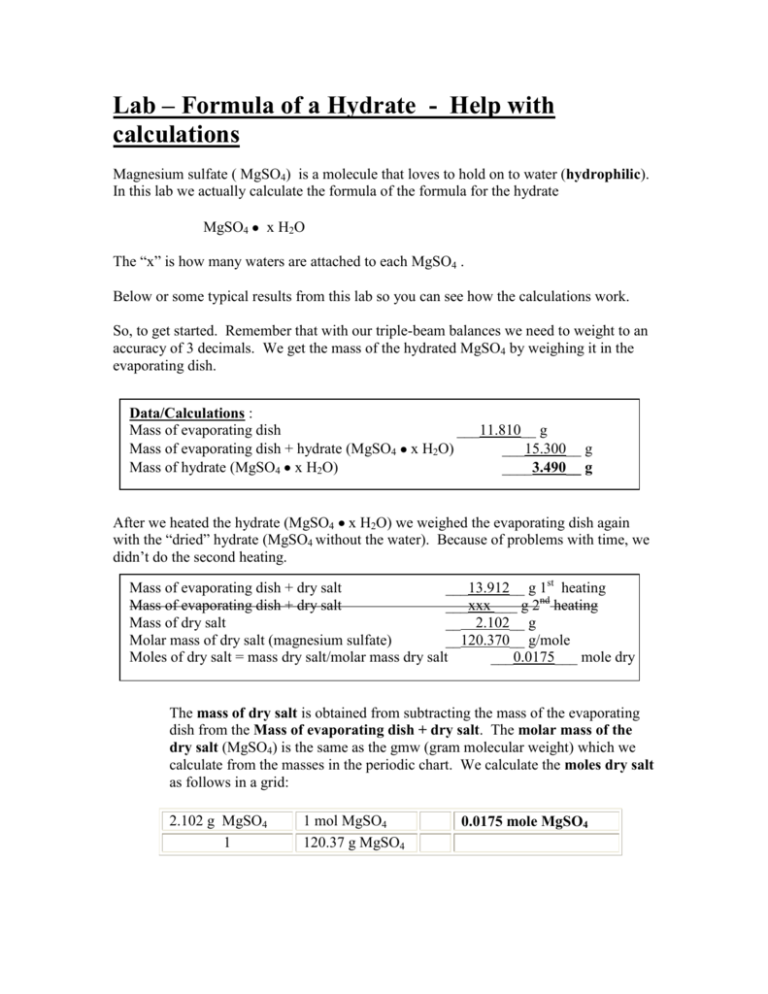
Lab – Formula of a Hydrate - Help with calculations Magnesium sulfate ( MgSO4) is a molecule that loves to hold on to water (hydrophilic). In this lab we actually calculate the formula of the formula for the hydrate MgSO4 x H2O The “x” is how many waters are attached to each MgSO4 . Below or some typical results from this lab so you can see how the calculations work. So, to get started. Remember that with our triple-beam balances we need to weight to an accuracy of 3 decimals. We get the mass of the hydrated MgSO4 by weighing it in the evaporating dish. Data/Calculations : Mass of evaporating dish ___11.810__ g Mass of evaporating dish + hydrate (MgSO4 x H2O) ___15.300__ g Mass of hydrate (MgSO4 x H2O) ____3.490__ g After we heated the hydrate (MgSO4 x H2O) we weighed the evaporating dish again with the “dried” hydrate (MgSO4 without the water). Because of problems with time, we didn’t do the second heating. Mass of evaporating dish + dry salt ___13.912__ g 1st heating Mass of evaporating dish + dry salt ___xxx ___ g 2nd heating Mass of dry salt __ 2.102__ g Molar mass of dry salt (magnesium sulfate) __120.370__ g/mole Moles of dry salt = mass dry salt/molar mass dry salt ___0.0175___ mole dry salt The mass of dry salt is obtained from subtracting the mass of the evaporating dish from the Mass of evaporating dish + dry salt. The molar mass of the dry salt (MgSO4) is the same as the gmw (gram molecular weight) which we calculate from the masses in the periodic chart. We calculate the moles dry salt as follows in a grid: 2.102 g MgSO4 1 1 mol MgSO4 120.37 g MgSO4 0.0175 mole MgSO4 Now that we know how many moles of MgSO4 we have, we can figure the moles of water that were with the salt. Mass of water = mass hydrate - mass dry salt Molar mass of water Moles of water = mass water/molar mass water ____1.388___ g ___18.0___ g/mole ____0.0771__ mole water Now we know the moles of water that we had attached to the MgSO4 molecules. We calculated the Molar mass of water (the gmw) using the periodic chart. Then we did the same type of calculation we did for the salt to figure the moles of the water as follows: 2.39 g H2O 1 1 mole H2O 18.0 g H2O 0.130 mole H2O Moles of water / mole dry salt = 0.0771 moles water/ _0.0175_moles dry salt = _4.4_: 1 (ratio) Mole ratio of dry salt to water: 1 mole dry salt: _4_mole water (ratio) Empirical formula of magnesium sulfate hydrate: MgSO4 _4_H20 (calculated from results) The two grids above calculated moles water and moles dry salt . The ratio the way we have been doing it: mole ratio 4 H2O : 1 MgSO4 When we put that into the formula we reverse the positions so the ratio reads: mole ratio 1 MgSO4 : 4 H2O That is why the formula for this hydrate, using the masses given above becomes MgSO4 4H2O (NOTE: This is not the correct formula for this hydrate!! The student did not weigh everything properly!!!!) So, congratulations, you have now calculated the formula for the hydrate of magnesium sulfate. You can buy this stuff in the grocery store! It is called Epsom Salts. The labeling of most Epsom salt containers will give you the formula of this hydrate.








