The molecular straucture of DNA
advertisement

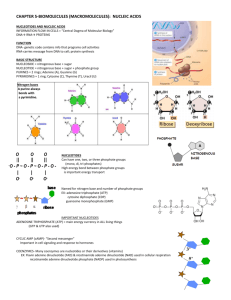
The molecular straucture of DNA A nucliec acid consists of a chemically linkid sequence of nucleotides. Each nucleotide contains heterocyclic ring of carbon and nitrogen atoms ( the nitrogenous base), a five – carbon sugar in ring form (a Pentose), and (a phosphate) group. The nitrogenous bases fall into two types Pyrimidines and purines. Pyrimidines have a six-member ring: purines have fused five – and six member rings. Each nucleic acid is synthesized from only four types of base . the same two purines, adenine (A) and guanine (G), are present in both DNA and RNA. The two pyrimidines in DNA are cytosine (C) and thymine(T).The only differences between uracil and thymine is the presence of methyl substituent at position C5. ; The sugar involved The sugar found in DNA is a five carbon sugar, otherwise known as a pentose sugar. These sugar can exist in two forms either as a ring structure or as a long ,five carbon chain. However , it is the ring form which is found here ,as depicted in the figure . One of the important points to see here is that the 2 ′ carbon (pronounced TwoPrime carbon) has no OH group attached to it, unlike the 1′ and 3 ′ carbons around the ring, and hence this sugar is called 2 ′ -deoxyribose .In RNA , the 2 ′ carbon does attach to an OH group, and here we can see one of the the major differences between DNA and RNA. r not The phosphate To complet the monomer structure requires the addition of a phosphate unit to the nucleoside. One this happens the whole monomer unit is reffered to as a nucleotide. In a nucleotide we find three phosphate groups which have been joined in a long chain, here we should note that the phosphate groups are labelled α ,β, and γ. DNA is a polymer of nucleotide units We can see that the unitary structure of DNA is the nucleotide monomer, made up of sugar, a phosphate chain and a base, where the base is any one of four.G, T,Aand C. Therefore, we have building blocks prepared and can now polymerize these to form a long chain, or polynucleotid. Here, the nucleotides are held together by the bonding of the 3′ carbon of the deoxyribose sugar to the first or α phosphate group of the next nucleotide. This bond is reffered to as phosphodiester bond, and results in the β, and γ phosphate groups of bonding phosphate chain being lost. Bis- phosphate by product is further cleaved to yield inorganic phosphatet Pi with a release of energy. Several important points need to be noted : As hinted at the earlier, the resulting polynucleotide has a direction to it, that is, the ends are not the same. At one end there is still a triphosphate unit attached to 5′ carbon of the sugar, and therefor, this end of the chain is called 5′ end ( pronounced five prime) . At the other end is an unused 3′ ( three prime) carbon still attached to an untouched OH group. This is the 3′ end of the chain. Further polymerization could still take place but, even if it did, we still end up with a 5′ end and a 3′ end.