ccnhccnh ch ch ooo - NAU jan.ucc.nau.edu web server
advertisement

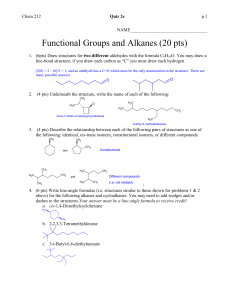
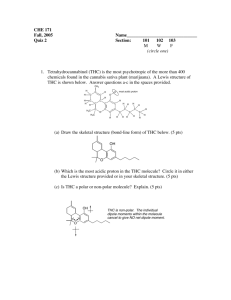
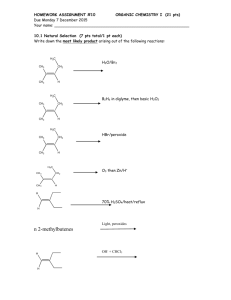
CHM235 Fall 05 September 21, 2005 Name on last page please! Exam 1 125 points + 1. (24 points) Lewis Structures and formula of organic molecules: (a) The skeleton of indole, the smell of fecal matter (stinky!!), is shown below. Add pi bonds and LP to make adenine the best Lewis Structure with no formal charges. Show all LP and pi bonds. 2 All NH bonds are shown. All Carbons are sp . N H What is the Molecular formula of indole? C__ H __ N __ (b) Phenobarbital, a drug prescribed for seizures in dogs, has the structure shown below: (All bonds correct). H N O 17 C 1 13 N H 14 What is the hybridization of C6. What are the /_C3C4C5 bond angle? Number of LP electrons O C 2 C 3 16 C H3C 6 CH2 5 7 4 O 15 What is the hybridization of C4? What are the /_N13C2C4 bond angle? pi bonds 2. (30 pts) The reaction of HCl (stomach acid) and bicarbonate is shown below. Some people unadvisably use this to relieve indigestion. (a) Draw the formulas for the products of the reaction: (don’t need Lewis structures here.) H-Cl + HCO3 (aq) + Hydrogen Chloride bicarbonate ion carbonic acid chloride ion Draw the Lewis structure of carbonic acid (carbonic acid is an O-H acid) Carbonic acid is unstable and breaks down rapidly into water and you burp.) (b) If the pKa of HCl = --5 and the pKa of carbonic acid = 7 and the pKa of H2O = 16. Which direction will the reaction proceed? What is the value of Keq? . (hint: This might make (c) Methyl isocyanate, CH3NCO, is a toxic substance responsible for the chemical accident in Bhopal India. Fill in the electrons on the skeleton of the main resonance structure of methylisocyanate. Complete the Lewis structures with all LP and pi bonds that will account for the main resonance structures of methyl isocyanate. Show all FORMAL CHARGES. NO formal charge fomal charges here H N Cb O H Ca N Cb O H Ca H H H hybridization of hybridization of Ca = Ca = N = N = Cb= Cb= O= O= Are these resonance forms significant (i.e. important) in explaining the structure of methyl isocyanate? Briefly explain (Hint: What shape would you predict for methylisocyanate ignoring methyl)) 3. (20 pts) Recognizing acids and bases: O O C O C Oxalic acid, HO C OH, has a pKa = 2 Methanol, CH3OH has a pKa = 16. + methanol (b) Label Acid, CA (conjugate acid), Base, CB (conjugate base). (c) Will this reaction be spontaneous as written? Yes or no Keq = (d) What is the name of the conjugate base of oxalic acid? C - O , has pKa = 4. Oxalate, HO + Methyl oxonium (like hydronium) ion, CH3OH2 has a pKa = -2. (a) Write the reaction for oxalic acid + methanol oxalic aicd O 4. (8 pts) Hydrogen bonding. For each pure substance indicate whether the substance can be either an H-bond donor, and H-boind acceptor, both or neither. H Cl O O H3C O CH3 Ethyl acetate (polish remover) Acceptor Donor Neither Both H3C acetone Acceptor Donor Neither Both O CH3 H H3C OH acetic acid Acceptor Donor Neither Both 4. (8 pts) Explain the following data: F H H Freon (air conditioning) Acceptor Donor Neither Both CH3 H3C Cl H C (a) Butyl chloride, 3 has a BP = 78*C tert-butyl chloride Briefly state which one is more volatile and why. Cl CH3 has a BP = 51*C H3C OH is infinitely soluble in water, but diethyl ether H3C (b) isobutyl alcohol H3C 7.5% soluble in water. Briefly state why. O CH3 is only 6. (6 points) Molecular Orbitals Label the following orbitals of carbon monoxide CO as σ, σ*, π, or π* based on the shapes and atomic orbitals involved. The bond axis is the C - O bond.. C O C Nodes Nodes are always perpendicular to the bond axis. O Nodes 7. (16 pts) Provide the best name for the following alkanes: H3C CH3 H3C H3C CH3 H3C CH3 CH3 CH3 CH3 H3C H3C CH3 CH3 H3C 8. (12 pts) Draw the following alkanes from name. 4-(1-methylethyl)octane 1-cyclobutylpentane 1,1,3,3-tetramethylcyclohexane 9. (12 points) Functional Groups: Circle and identify all the functional groups below. NO ALKANES PLEASE. Levetiracetam (brand name: Keppra®) an anticonvulsant medication Haloperidol (Haldol®) antipsychotic ® Loxapine (trade name Loxitane ) Name: Points from Page 1 out of 38 points. Page 2 out of 34 points. Page 3 out of 30 points. Page 4 out of 35 points. Total Total Points 137 points out of 125 points.