Chemistry 20 Chapter 4 Benzene
advertisement

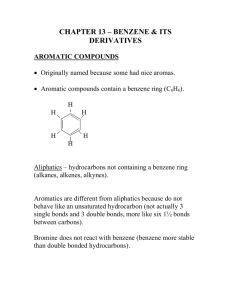
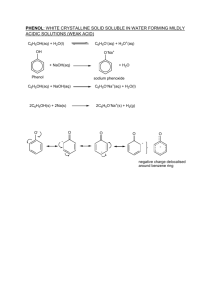
Chemistry 20 Chapter 4 Benzene Benzene: a molecule of benzene consists of a ring of six carbon atoms with onr hydrogen atom attached to each carbon. It has the molecular formula C6H6. The real molecule of benzene is a resonance hybrid of the two Lewis structures (a unique feature that makes benzene chemically stable). H H C C H C C H C C H H H H C C H C C H C C H H Aromatic compounds: they are unsaturated hydrocarbons that contain one or more benzene rings in their structures. Because some of them have pleasant odors, they were called aromatic compounds. Arene: a compound containing one or more benzene-like rings. Aryl group: a group derived by removal of an H atom from an arene is called an arel group and given the symbol Ar- (phenyl C6H5-) Naming aromatic compounds: 1. When one substituent group is attached to benzene, we write the name of the group in front of benzene. CH2 CH3 Cl + NO2 + Ch lorobenzen e N itrob enzene Eth ylb enzene Note: The IUPAC system retains certain common names for several of the simpler monosubstituted alkylbenzenes: CH3 Toluen e OH Phen ol OCH3 A nisole NH2 A niline O C-H Ben zaldehyde O C-OH Benzoic acid 2. When two or more substituents are attached to benzene, the ring is numbered give the lowest numbers to the substituents. The substituents are listed alphabetically. Dr. Behrang Madani Chemistry 20 Mt SAC Note: When two substituents occur on a benzene ring, three isomers are possible. We locate the substituents either by numbering the atoms of the ring or by using the locators ortho (o), meta (m), and para (p). 1 4 1 2 3 1 1,2- or 1,3- or meta 1,4- or para ortho- 1 COOH Br 2 1 CH2 CH3 CH3 4 3 2 3 2 CH3 2-Bromobenzoic acid (o-Bromoben zoic acid) 1 Cl 1-Ch loro-4-ethylbenzen e (p-Chloroeth ylb enzene) 1,3-D imethylben zene (m-Xylen e) Note: The substituent group derived by loss of an H atom from benzene is called a phenyl group (C6H5-). 4 C6 H5 1-Phenylcyclohexene 3 2 1 4-Phenyl-1-butene Resonance: although, benzene has three double bonds, it does not behave like an alkene. We can draw two Lewis structures for benzene (with two different positions for its double bonds). Each individual Lewis structure is called a contribution structure. The real molecule of benzene is a resonance hybrid of these two Lewis structures. Therefore, the carbon-carbon bonds are neither single nor double but rather something intermediate between the two extremes. Whenever we find resonance, we find stability. Benzene ring is greatly stabilized by resonance, which explains why it does not undergo the addition reactions typical of alkenes. H H C C H C C H C C H H H H C C H C C H C C H H Chemical properties of benzene and its derivatives: The most important characteristic reaction of aromatic compounds is substitution at a ring carbon (aromatic substitution). We can name for them these three reactions: 1.Halogenation 2. Nitration 3. Sulfonation. Halogenation: in the present of an iron catalyst, chlorine reacts rapidly with benzene to give chlorobenzene and HCl. Dr. Behrang Madani Chemistry 20 Mt SAC FeCl3 H + Cl2 Cl + HCl Ch lorobenzen e Benzen e Nitration: when we heat benzene or one of its derivatives with a mixture of concentrated nitric and sulphuric acids, a nitro (-NO2) group replaces one of the hydrogen atoms bonded to the ring. In this reaction, sulfuric acid is a catalyst and it is added to speed up the reaction. H2 SO4 H + HNO3 NO2 + H2 O N itrob enzene Sulfonation: By heating an aromatic compound with concentrated sulfuric acid, one of the H atom is replaced by sufonic acid (-SO3H) group (all of which are strong acids, comparable in strength to sulfuric acid). H + H2 SO4 SO3 H + H2 O Benzenes ulfon ic acid Phenol: a compound that contains an hydroxyl group (-OH) bonded to a benzene ring. Substituted phenols are named either as derivatives of phenol or by common names. OH OH 1 3 3 -M e th y l p h e n o l (m - C re s o l ) (m-Cresol) OH 2 1 2 NO2 3 Cl 3-Chloro-2-nitrophenol Phenol Note: Phenols are widely distributed in nature. Many of them are used as antiseptics and disinfectants. Phenols are weak acids, with pKa values of approximately 10. Most phenols are insoluble in water, but they react with strong bases, such as NaOH and KOH, to form watersoluble salts. OH + NaOH Phenol H2 O O- Na + + H2 O S od ium phenoxide (a w ater-soluble salt) Autoxidation: foods and other materials that contain C=C can be oxidized only in presence of oxygen (no other reactant). This oxidation is called Attoxidation. In this reaction, an R-H group is converted to an R-O-O-H (hydroperoxide) group. H CH2 CH=CH-CH Section of a fatty acid h yd rocarbon chain Dr. Behrang Madani + light O2 or h eat Oxygen Chemistry 20 O-O-H CH2 CH=CH-CH-CH2 A hydroperoxid e Mt SAC Note: Phenols are antioxidants and they can prevent autoxidation. Therefore, they are added to foods to “retard spoilage”. For example, Vitamin E as a phenol is an antioxidant. O H 3 HO Vitamin E Dr. Behrang Madani Chemistry 20 Mt SAC