14.2
The Gas Laws
>
Boyle’s Law: Pressure and Volume
Boyle’s Law: Pressure and Volume
How are the pressure, volume, and
temperature of a gas related?
Slide
1 of 45
© Copyright Pearson Prentice Hall
14.2
The Gas Laws
>
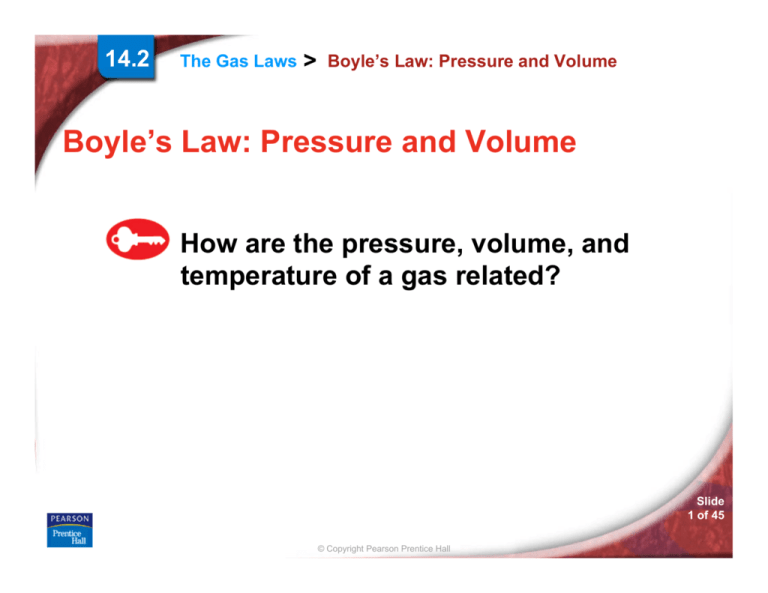
Boyle’s Law: Pressure and Volume
If the temperature is constant, as
the pressure of a gas increases, the
volume decreases.
Slide
2 of 45
© Copyright Pearson Prentice Hall
14.2
The Gas Laws
>
Boyle’s Law: Pressure and Volume
Boyle’s law states that for a given mass of gas
at constant temperature, the volume of the gas
varies inversely with pressure.
Slide
3 of 45
© Copyright Pearson Prentice Hall
14.2
The Gas Laws
>
Boyle’s Law: Pressure and Volume
Slide
4 of 45
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 14.1
Slide
5 of 45
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 14.1
Slide
6 of 45
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 14.1
Slide
7 of 45
© Copyright Pearson Prentice Hall
Practice Problems for Sample Problem 14.1
Problem Solving 14.8
Solve Problem 8 with the help
of an interactive guided
tutorial.
© Copyright Pearson Prentice Hall
Slide
8 of 45
14.2
The Gas Laws
>
Charles’s Law: Temperature and Volume
Charles’s Law: Temperature and Volume
As the temperature of an enclosed gas
increases, the volume increases, if the
pressure is constant.
Slide
9 of 45
© Copyright Pearson Prentice Hall
14.2
The Gas Laws
>
Charles’s Law: Temperature and Volume
As the temperature of the water increases, the
volume of the balloon increases.
Slide
10 of 45
© Copyright Pearson Prentice Hall
14.2
The Gas Laws
>
Charles’s Law: Temperature and Volume
Charles’s law states that the volume of a fixed
mass of gas is directly proportional to its Kelvin
temperature if the pressure is kept constant.
Slide
11 of 45
© Copyright Pearson Prentice Hall
14.2
The Gas Laws
>
Charles’s Law: Temperature and Volume
Slide
12 of 45
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 14.2
Slide
13 of 45
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 14.2
Slide
14 of 45
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 14.2
Slide
15 of 45
© Copyright Pearson Prentice Hall
Practice Problems for Sample Problem 14.2
Problem Solving 14.10
Solve Problem 10 with the help
of an interactive guided tutorial.
Slide
16 of 45
© Copyright Pearson Prentice Hall
14.2
The Gas Laws
>
Gay-Lussac’s Law: Pressure and Temperature
Gay-Lussac’s Law: Pressure and
Temperature
As the temperature of an enclosed gas
increases, the pressure increases, if the
volume is constant.
Slide
17 of 45
© Copyright Pearson Prentice Hall
14.2
The Gas Laws
>
Gay-Lussac’s Law: Pressure and Temperature
When a gas is heated at constant volume, the
pressure increases.
Slide
18 of 45
© Copyright Pearson Prentice Hall
14.2
The Gas Laws
>
Gay-Lussac’s Law: Pressure and Temperature
Gay-Lussac’s law states that the pressure of a gas
is directly proportional to the Kelvin temperature if
the volume remains constant.
Slide
19 of 45
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 14.3
Slide
20 of 45
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 14.3
Slide
21 of 45
© Copyright Pearson Prentice Hall
Practice Problems for Sample Problem 14.3
Problem Solving 14.12
Solve Problem 12 with the
help of an interactive guided
tutorial.
© Copyright Pearson Prentice Hall
Slide
22 of 45
14.2
The Gas Laws
>
The Combined Gas Law
The combined gas law describes the relationship
among the pressure, temperature, and volume of an
enclosed gas.
Slide
23 of 45
© Copyright Pearson Prentice Hall
14.2
The Gas Laws
>
The Combined Gas Law
The combined gas law allows you to do
calculations for situations in which only
the amount of gas is constant.
Slide
24 of 45
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 14.4
Slide
25 of 45
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 14.4
Slide
26 of 45
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 14.4
Slide
27 of 45
© Copyright Pearson Prentice Hall
Practice Problems for Sample Problem 14.4
Problem Solving 14.14
Solve Problem 14 with the help
of an interactive guided tutorial.
© Copyright Pearson Prentice Hall
Slide
28 of 45