1 Youngstown State University Department of Chemistry Chemistry
advertisement

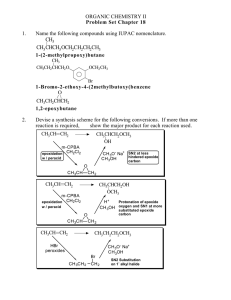
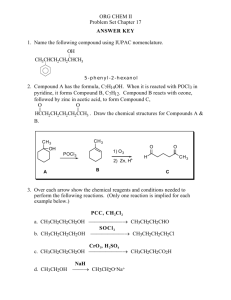
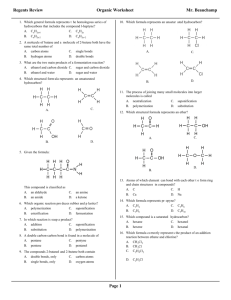
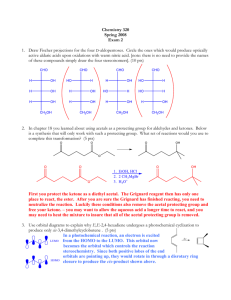
Chemistry 1506 Practice Exam 1 Youngstown State University Department of Chemistry Chemistry 1506 Practice Exam 1 25 Questions, 4 points each 1. What is the correct IUPAC name for the following compound? CH 2 CH 2CH 3 CH 3 C CH 3 CH 3 (a) 2-methylisopentane (b) 2,2,-dimethylisopentane (c) 2,2,-dimethylpentane (d) t-butylpropane 2. Which structure corresponds to the name 1,4-diethylcyclohexane? (a) (b) CH 2 CH 2 CH 3 CH 2 CH CH 3CH 2 CH CH CH 2CH 3 (d) CH 3 CH 2 CH CH 3CH 2 CH 2 CH 2 CH 2 CH 2 CH 2 1,4-diethylcyclohexane CH CH CH 2 CH 2 CH 2 (c) CH 2CH 3 CH 2 CH CH 2 CH CH 2CH 3 CH 2 CH 2 CH 2 CH 2 CH 3 CH 2 3. How many O2 molecules are required to balance the complete oxidation of cyclobutane as shown below? CH 2 CH 2 + (a) 2 CO 2 O2 CH 2 CH 2 (b) 4 (c) 6 + H 2O (d) 8 4. How many geometric isomers are possible for the compound whose formula is C2H2Cl2, one of which is shown below? Cl C CH 2 Cl (a) 1 (b) 2 (c) 3 (d) 4 5. Saturated hydrocarbons undergo what reaction in biological systems? (a) bromination (b) oxidation (c) hydration (d) none 1 Chemistry 1506 Practice Exam 1 6. Which name is correct? (a) 2,3-dibromocyclobutane (c) 1,2-dimethylcyclopropane (b) 1,4-diethylcyclobutane (d) 4,5,6-trichlorocyclohexane 7. Which structure is incorrect? (a) CH 3 CH 3 CH (c) Br CH 2 CH 2 CH 3 CH 3CH 2 (b) CH 2 CH 3 Br H CH 3CH 2 C CH 3 Br (d) Br Br CH 3CHCH 2 C CH 3 Br 8. When writing the C=C, both bonds appear the same, however we know in fact that: (a) there is free rotation around it. (c) one is a σ bond and the other is a π bond (b) one is straight and the other is bent. (d) they are both p bonds. 9. If water were added to the olefin below, in dilute H+, what would be the name of the major product? CH 3 CH 2 C CHCH 3 + (a) 2-ethyl-3-pentanol (b) 2-ethyl-2-pentanol (c) 3-ethyl-2-pentanol (d) 3-ethyl-3-pentanol H 2O CH 3 CH 2 10. What would be the name of the hydrocarbon resulting from the addition of H2 (over Ni) to the following olefin? CH CH 3 CH CH CHCH 3 + H2 CH 2 CH 2 (a) 2,4-dimethylcyclohexane (b) 1,5-dimethylcyclohexane (c) 3,6-dimethylcyclohexane (d) 1,4-dimethylcyclohexane 11. What is the IUPAC name of the following olefin? CH 3CH 2 CH CHCHCH 2 CH 3 Br (a) 3-bromo-5-heptene (b) 5-bromo-3-heptene (c) 3-bromo-4-heptene (d) 4-bromo-3-heptene 2 Chemistry 1506 Practice Exam 1 12. Which of the following formulas corresponds to cis-3-octene? (b) (a) CH 3CH 2 CH 2CH 2CH 3 CH 3CH 2 CH 2 C C H H C C H H CH 2CH 2CH 3 (d) (c) CH 3CH 2 CH 2CH 2 CH 3CH 2 CH 2CH 3 C C H C C H H H CH 2CH 2CH 2 CH 3 13. Which of the following is a secondary (2o) alcohol? (a) (b) CH 3CH 2 OH CH 3CH 2 CHCH 2 CH 3 (c) CH (d) CH 3CHCH 2OH CH CH 3 OH CH CH C OH CH 14. Name the product of the following reaction. (a) 1-propanol (b) 2-propanol (c) 1-butanol (d) 2-butanol H+ CH 3CH 2 CH CH 2 + H 2O 15. What product would result from the oxidation of cyclohexanol (below). CH 2 CH 2 CH 2 (a) (b) (c) (d) [O] CH OH CH 2 CH 2 cyclohexene cyclohexane cyclohexanone cyclohexanal 16. Which compound would fail to react with oxidizing agents MnO4- and Cr2O72-? OH (a) CH 3OH (b) CH 3CH 2OH (c) CH 3CHCH 3 OH (d) CH 3 CCH 3 CH 3 3 Chemistry 1506 Practice Exam 1 17. Choose whether 2-methyl-2-pentanol is 1o, 2o, 3o, or 4o. OH CH 3CH 2CH 2 CCH 3 (a) 1o CH 3 (b) 2o (c) 3o (d) 4o 18. What product (if any) would result from the oxidation of 2-methyl-2-hexanol? (a) 2-methyl-2-hexanone (b) 2-methyl-2-hexanal (c) 2-methyl-2-hexene (d) No Reaction 19. What is the organic product of a reaction of ethanal with Tollen’s reagent (Ag+/OH-/NH3)? [Ag metal forms a silver mirror as Ag+ is reduced]. O O (a) (b) CH 3C O (c) CH 3C CH 3C OH H O - (d) No Reaction 20. What is the organic product of a reaction of acetone (2-propanone) with Benedict’s reagent (Cu2+/OH-)? OH OH O OH (a) CH 3 CHCH 3 (b) CH 3 CCH 3 (c) CH 3 CH CH 2 (d) No Reaction 21. To convert ethanal to ethanol, one must do a(an) (type of reaction)? (a) oxidation (b) reduction (c) addition (d) condensation 22. What aldehyde and alcohol were used to form the following hemiacetal? (a) (b) (e) (f) OH CH 3 CH OCH 3 acetaldehyde and methanol acetaldehyde and ethanol formaldehyde and methanol formaldehyde and ethanol 23. What reaction would lead to the following product? OH O CH 3CH 2C CHC H H CH 3 (a) (b) (c) (d) oxidation of 1-hexanol reduction of 3-hexanone aldol condensation of propanal ketal condensation 24. What is the molecular formula of glycerol, 1,2,3-trihydroxypropane? (a) C3H6O3 (b) C3H8O3 (c) C3H9O3 (d) C3H10O3 25. How many carbon atoms are there in a molecule of 1,2,5-triisopropylcyclohexane? (a) 9 (b) 12 (c) 15 (d) 18 4