CHEMICAL ELEMENTS THAT MAKE UP Carbohydrates Carbon
advertisement

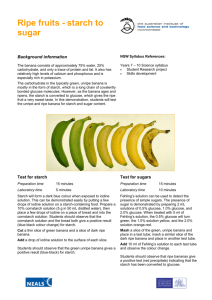
CHEMICAL ELEMENTS THAT MAKE UP Carbohydrates Carbon, hydrogen (2), oxygen = CH2O Fats Carbon, hydrogen, oxygen Proteins Carbon, hydrogen, oxygen, nitrogen, sulphur MONOSACCARIDE DISACCARIDE POLYSACCARIDE glucose maltose starch AMINO ACIDS DIPEPTIDE POLYPEPTIDE 1 amino acid 2 amino acids e.g. protein CARBOHYDRATES PROTEINS peptide bonds join amino acids together 3 fatty acid molecules and 1 glycerol molecule form a lipid. Lipids come in three forms: liquid (oils), semi-solid (wax) and solid (fats) Principal source: Carbohydrates bread, rice, potatoes, pasta, cereal Fats meat, dairy products, nuts Proteins meat, fish, cheese, vegetables fresh fruits and vegetables Vitamins C Vitamin D Certain types of fish sunlight Calcium cows’ milk, vegetables Iron breakfast cereals, liver, meat, nuts, green vegetables wholemeal bread and pasta, fruits and vegetables, nuts, seeds Fibre Water Importance: Carbohydrates are the body’s main source of fuel. They give us our energy. They’re needed for our body to function properly Energy, absorbing certain vitamins, maintaining cell membranes Tissue growth and repair, maintaining immune system, making essential hormones and enzymes It can protect cells from damage, it can prevent cataracts. A lack of vitamin C can cause scurvy. Promotes calcium absorption in the gut and bone growth. A lack of vitamin D can cause bones to become thin and brittle, and can cause rickets. For bone growth, for muscles to contract and relax properly. Lack of calcium can cause rickets. Helps make red blood cells (key component of haemoglobin). Lack can cause anaemia, fatigue and hair loss. Aids digestion but doesn’t get digested It is necessary for the digestion and absorption of food; helps maintain proper muscle tone; supplies oxygen and nutrients to the cells; rids the body of wastes TEST FOR: Starch IODINE A solution or solid can be tested for starch by adding iodine to it. If the solution or solid (such as a potato) turns black-blue, starch is present. If no starch is present, the iodine remains the colour it is. Reducing BENEDICT’S A food product can be tested for reducing sugars such as glucose or sugars REAGENT lactose. Dissolve a food sample in boiling water. Add a small amount of Benedict’s reagent. Over the next four to ten minutes, as the solution cools, it should begin to change colour. If glucose is present, the colour will change to green, yellow, orange or red/brown (from very little to a lot of glucose present in the solution) Protein AQUEOUS To test whether a mixture contains protein, or peptide bonds, add a COPPER(II) small amount of the mixture to a test tube along with an equal amount SULPHATE of a strong base (e.g. sodium hydroxide, potassium hydroxide, etc.). Mix these together, and add a few drops of aqueous copper(II) sulphate. Swirl the mixture around; if protein is present, the mixture will turn purple. If no protein is present, the mixture will turn blue. Fats ETHANOL To test for fat in a solution, add 2cm3 of ethanol to a few drops of the solution in a test-tube, and shake the mixture well. Add 2cm3 of water. If the solution contains fat, a cloudy white suspension will form at the top of the solution. Microorganisms are used in the manufacture of yoghurt for the formation of typical yoghurt flavour and texture.