Analysis of Gasoline Using a GC-MS
advertisement
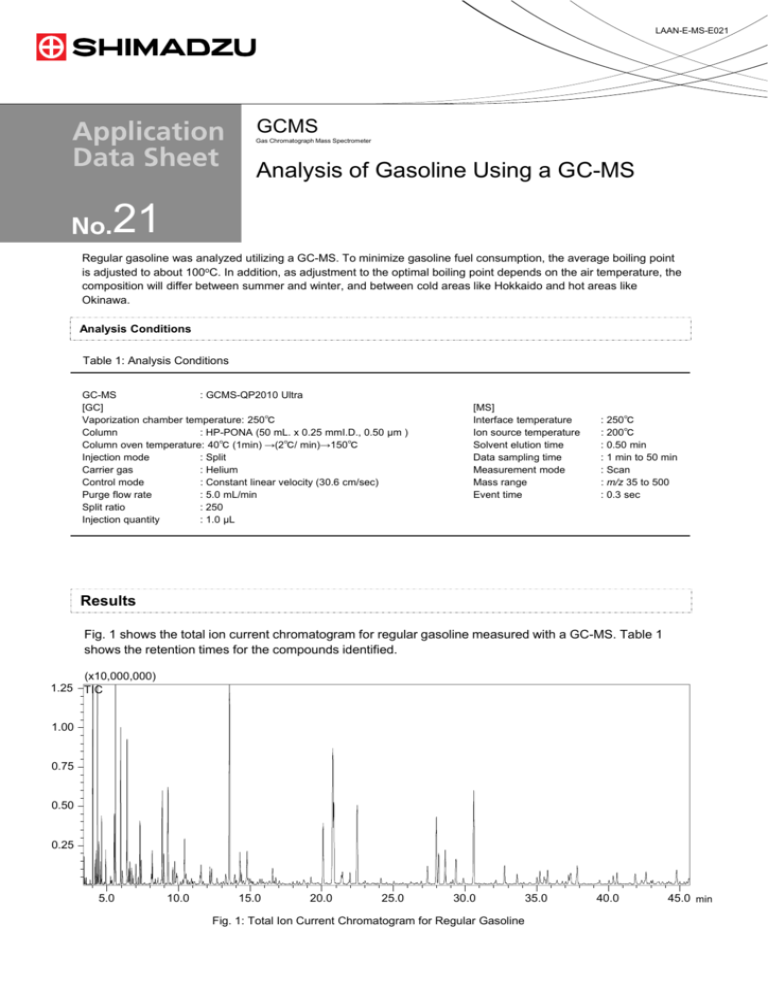
LAAN-E-MS-E021 GCMS Gas Chromatograph Mass Spectrometer Analysis of Gasoline Using a GC-MS 21 Regular gasoline was analyzed utilizing a GC-MS. To minimize gasoline fuel consumption, the average boiling point is adjusted to about 100oC. In addition, as adjustment to the optimal boiling point depends on the air temperature, the composition will differ between summer and winter, and between cold areas like Hokkaido and hot areas like Okinawa. Analysis Conditions Table 1: Analysis Conditions GC-MS : GCMS-QP2010 Ultra [GC] Vaporization chamber temperature: 250℃ Column : HP-PONA (50 mL. x 0.25 mmI.D., 0.50 µm ) Column oven temperature: 40℃ (1min) →(2℃/ min)→150℃ Injection mode : Split Carrier gas : Helium Control mode : Constant linear velocity (30.6 cm/sec) Purge flow rate : 5.0 mL/min Split ratio : 250 Injection quantity : 1.0 µL [MS] Interface temperature Ion source temperature Solvent elution time Data sampling time Measurement mode Mass range Event time : 250℃ : 200℃ : 0.50 min : 1 min to 50 min : Scan : m/z 35 to 500 : 0.3 sec Results Fig. 1 shows the total ion current chromatogram for regular gasoline measured with a GC-MS. Table 1 shows the retention times for the compounds identified. (x10,000,000) 1.25 TIC 1.00 0.75 0.50 0.25 5.0 10.0 15.0 20.0 25.0 30.0 Fig. 1: Total Ion Current Chromatogram for Regular Gasoline 35.0 40.0 45.0 min 21 Table 1: List of Identification Results for Regular Gasoline Retention time (mm) Retention Time 3.240 3.349 3.412 3.467 3.564 3.834 4.020 4.180 4.265 4.329 4.429 4.548 4.625 4.911 5.259 5.304 5.345 5.503 5.518 5.597 5.953 6.070 6.101 6.407 6.452 6.492 6.534 6.604 6.719 6.831 7.024 7.193 7.301 7.390 7.623 7.988 8.113 Compound Name Isobutane 2-buten n-butane trans-2-buten cis-2-buten 3-methyl-1-buten Isopentane 1-pentene 2-methyl-1-buten n-pentane trans-2-pentene cis-2-pentene 2-methyl-2-buten 2,2-dimethylbutane Cyclopentene 4-methyl-1-pentene 3-methyl-1-pentene cyclopentane 2,3-dimethylbutane 2-methylpentane 3-methylpentane 2-methyl-1-pentene 1-hexene n-hexane trans-3-hexene cis-3-hexene trans-2-hexene 2-methyl-2-pentene cis-3-methyl-2-pentene cis-2-hexene trans-3-methyl-2-pentene 2,2-dimethylpentane Methylcyclopentane 2,4-dimethylpentane 2,2,3-methylbutan 2,4-dimethyl-1-pentene 1-methylcyclopentane Retention Time 8.160 8.382 8.563 8.533 8.708 8.800 8.864 8.968 9.129 9.257 9.598 9.731 10.144 10.297 10.410 10.538 10.561 10.644 10.754 10.903 11.086 11.501 11.564 12.186 12.309 12.685 13.394 13.563 13.935 14.039 14.212 14.286 14.387 14.782 15.681 15.839 16.432 Compound Name Benzene 3,3-dimethylpentane Cyclohexane cis-2-methyl-3-hexene 4-methyl-1-hexene 4-methyl-2-hexene 2-methylhexane 2,3-dimethylpentane Cyclohexene 3-methylhexane trans-1,3-dimethylcyclopentane cis-1,3-dimethylcyclopentane cis-3-methyl-3-hexene trans-3-heptene n-heptane 2-methyl-2-hexene cis-3-methyl-2-hexene trans-2-heptene 3-ethyl-2-pentene trans-3-methyl-2-hexene cis-2-heptene 1,2-dimethylcyclopentane Methylcyclohexane 2,5-dimethylhexane 2,4-dimethylhexane 1,2,4-trimethylcyclopentane 3-ethylcyclopentene Toluene 2,3-dimethylhexane 2-ethyl-3-methyl-1-pentene 1-methylcyclohexane 2-methylheptane 4-methylheptane 3-methylheptane 1-ethyl-3-methylcyclopentane 1-ethyl-3-methylcyclopentane 4-octene Retention Time 16.560 19.239 20.084 20.737 20.816 21.376 21.452 21.973 22.487 24.130 27.388 28.031 28.189 28.641 29.147 29.398 29.889 30.623 32.167 32.770 33.640 35.017 35.238 35.535 35.768 36.440 37.034 37.244 37.381 40.061 40.354 40.626 41.875 42.643 44.788 Compound Name n-octane 2,5-dimethylheptane Ethylbenzene m-xylene p-xylene 4-methyloctane 2-methyloctane 3-methyloctane o-xylene n-nonane n-propylbenzene m-ethyltoluene p-ethyltoluene 1,3,5-trimethylbenzene 4-methylnonane o-ethyltoluene 3-methylnonane 1,2,4-trimethylbenzene n-decane 1,2,3-trimethylbenzene Indan 1,3-diethylbenzene 1-methyl-3-propylbenzene 1-methyl-4-propylbenzene 1,3-dimethyl-5-ethylbenzene Methyldecane Methyldecane 1,4-dimethyl-2-ethylbenzene 1,2-dimethyl-4-ethylbenzene n-undecane 1,2,4,5-tetramethylbenzene 1,2,3,5-tetramethylbenzene 5-Methylindan 2-Methylindan Naphthalene For Research Use Only. Not for use in diagnostic procedures. Shimadzu Corporation (“Shimadzu”) reserves all rights including copyright in this publication. Shimadzu does not assume any responsibility or liability for any damage, whether direct or indirect, relating to, or arising out of the use of this publication. This publication is based upon the information available to Shimadzu on or before the date of publication, and subject to change without notice. First Edition: October 2011