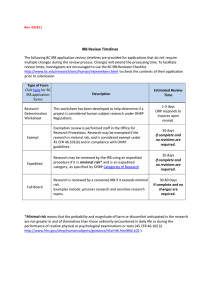
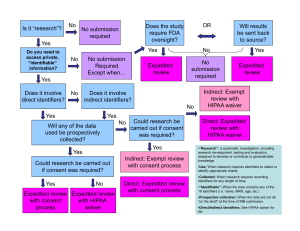
Drexel Non-Medical/Medical IRB Submission Flowchart WHAT IS MY LEVEL OF SUBMISSION?
advertisement

Drexel Non-Medical/Medical IRB Submission Flowchart http://www.research.drexel.edu/compliance/IRB/non_medical_irb.aspx WHAT IS MY LEVEL OF SUBMISSION? • • Release Letter No interaction with human subjects or privately held material Examples: Policy brief, literature review, US census data WHAT FORMS DO I NEED? Exempt Category 1 (link to form) - Research on regular and special education instructional strategies, comparison of instructional techniques, curricula, classroom management methods • • • • • • • • • • • • Exempt Review Less than “minimal risk”* to human subjects Example: Anonymous response survey, focus group or questionnaire Working with a completely deidentified (anonymous) secondary data set Expedited Review “Minimal risk”* to human subjects Minimal risk studies involving Vulnerable Populations** that may benefit the subject Examples: Prospective or retrospective chart review, surveys/questionnaires collecting limited personal identifiers or health information, any intervention or interaction with the subject Full Review More than “minimal risk”* to participants Any research project involving human subjects not covered under other review categories Contact with vulnerable populations with no potential for direct benefit Working with data that can be traced or linked to individual participants Interventions involving physical or emotional discomfort • • Letter of Determination 1-2 page study description written by student Exempt Category 2 (link to form) - Research on educational tests, survey/interview procedures, subjects must be deidentified, no vulnerable populations, must be “benign research” (no risk of liability or damage due to release of data) Exempt Category 3 (link to form) - Participants are appointed public officials or candidates for public office Exempt Category 4 (link to form) - Collection or study of existing publicly available deidentified data or specimens Exempt Category 6 (link to form) - Taste and food quality evaluation and consumer acceptance studies • Submit to Jack Medendorp for review ASAP Office of Regulatory Research Compliance (ORRC) informs student re: approval status If approved, ORRC sends student Release Letter All Submissions: • Proposal Submission Checklist [ MS Word ] • Project Submission Transmittal Form [ PDF ] – only required if there is external funding • Co-Investigator Form (if more than 2 co-investigators) [ MS Word ] • Research Proposal (sections addressing data collection/analysis, participant enrollment only) • Data Collection Instruments (surveys, questionnaires, advertisements etc.) Additional Forms for Exempt Review (submit original and two copies of all forms) • Conflict of Interest Form [ MS Word ] Additional forms for Expedited Review (submit original and three copies of all forms) • Expedited Review Application for Research involving Pathological Samples with Identifiers (if applicable) • Expedited Review for Charts (if applicable) [ MS Word ] • Expedited Certification Form [ MS Word ] Additional forms for Expedited and Full Review (Submit original and six copies of all forms for Full review) • Full Review/Expedited Review Combined Form [ MS Word ] • Conflict of Interest Form (Use this form if there is more than 1 collaborator who has a conflict of interest) [ MS Word ] • Informed Consent Non-medical Without HIPAA Authorization [ MS Word ] • Assent Form Template (for participation of children and minors) [ MS Word ] • Request for Consent Waiver (to waive or alter informed consent) [ MS Word ] • Internal Indemnification Form (for studies with no external sponsor, not applicable for chart reviews) [ MS Word ] • HIPPA Waiver of Authorization (access protected health information without participant consent) [ PDF ] • Investigators brochure or pertinent information documents (Patient Information Supplement) if research involves the use of a drug/device/interventional procedure *”Minimal risk” means that the risks of harm anticipated in the proposed research are not greater, considering probability and magnitude, than those ordinarily encountered in daily life or during the performance of routine physical and physiological examinations or tests (noninvasive examples, physical, blood pressure, EKG). Note that all proposals must include a risk/benefit assessment to identify whether the research will pose more than a "minimal risk" to the subject. **"Vulnerable Populations” include those whose interests require special protection, e.g., Pregnant women/fetus, prisoners, minors, subjects with psychiatric/mental disorders, etc. • • • IMPORTANT Example submissions available from Director of Community Projects (DCP) Copies of all approval paperwork go to DCP Review all paperwork carefully before submitting paperwork to IRB