Chemistry 2301 Thursday, December 5 At
advertisement

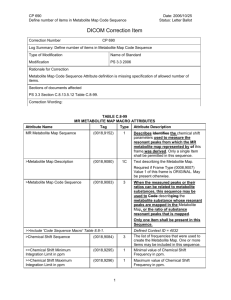
Chemistry 2301 Thursday, December 5 At-Home Exercise: IR Spectroscopy and MS Fragmentation Patterns Cladosporium resinae, sometimes called “fuel fungus”, colonizes aircraft fuel lines in wet climates. To use aircraft fuel as food, the fungus must first oxidize the fuel’s component hydrocarbons. To better understand this process, C. resinae was grown on n-pentane and oxygen, and initial metabolites were collected and analyzed by IR and MS. In this problem, you will try to determine the structure of one metabolite from IR and mass spectra. a. The IR spectrum of one pentane metabolite is shown below. Transmittance (%) 100 50 0 4000 3000 2000 1500 1000 500 Wavenumbers (cm-1) Hydrocarbon oxidation could presumably generate any of the functional groups shown below. Based on the IR spectrum, which would you predict to find in this metabolite? Circle all answers that apply. H C (sp) C(sp 2) H H C(sp 3) H O C C O C b. Let’s assume that the metabolite still has the linear, five-carbon skeleton of npentane. Given your answers to part (a), what are some possible structures for the molecule? c. The mass spectrum of the metabolite is shown below. Relative Intensity 100 45 80 60 40 88 20 73 0 10 20 30 40 50 60 70 80 90 100 m/z Let’s assume that the parent mass for [M+] has m/z = 88. Is this consistent with any of the structures you drew in part (b)? (Do any of them have mass 88 amu?) d. Which of your proposed structures is also consistent with fragment masses of m/z = 45 and 73 amu? How would your molecule have to fragment to generate these masses? e. In the mass spectrum, the peak at m/z = 45 is much taller than the peak at m/z = 73. Why do you suppose the m/z = 45 fragment is produced so much more than the m/z = 73 fragment?