208 MICROBIOLOGY
advertisement

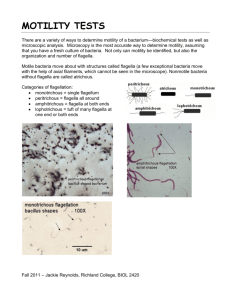
208 MICROBIO LOGY SECTION 1 LABORAT OR Y WEE K 2 - MOTILITY, BACTERIAL FLAGELLA MOTILITY, TRICHOMONAS VAGINALIS EX ER CIS E 3. THE WET MOUNT PREPARATION BACTERIAL SHAPES E X ER CI SE 4. SIMPLE STAINS MOT IL ITY Motility: Capable of or demonstrating movement by independent means Not e. True, inde pen de nt mot ility of bac ter ia gen era lly dep en ds on t heir posse ssion of fla ge lla. If so eq uip ped, th ey ca n pro pe l the mselv es wit h progre ssive , d irec tio na l loco mot ion . This kind of ac tive mo tio n must be dist in gu ishe d fro m BR OW NI AN MOVEMENT. Th is type of move me n t is a vibrat ory mo tio n of orga n isms and ot her part ic le s su spe nd ed in f lu id. Motile bacteria move using flagella, threadlike projections that extend out from the cell wall. Bacterial flagella are so thin that they cannot be visualized with brightfield microscopy. Flagellar ultrastructure is fundamentally different in the eukaryotes from the prokaryotes. Different bacterial species have distinctive arrangements of flagella. These characteristics can be exploited in the identification of infectious agents that may be involved in a given disease process. Below you will see basic arrangements for many prokaryotic microorganisms. Mo notr ichou s distribution means that each cell has a single flagellum. If the flagellum is located at the end of the cell, the cell is said to have monotrichous polar distribution, as is the case with Pseudomonas sp. Amp h itr ichou s bacteria have a single flagellum at each pole. Lopho tr ichou s distribution is a pattern in which bacteria appear to have a tuft ("lopho") of hair ("trichous") at one or both ends. Per itr icho us flagella are distributed uniformly over the surface of each bacterial cell. This pattern is characteristic for highly motile organisms like Proteus vulgaris . 2-1 A fourth type of flagellar distribution is the axial filament characteristic of the Spirochetes. Hundreds of individual periplasmic flagella are bundled together to create a struture that allows spinning and flexing motility. We will discuss this group in laboratory exercise 30. SPIROCHETE WITH AXIAL FILAMENT The major infectious agents which cause vulvo-vaginitis are Gardnerella vaginalis, Candida albicans and Trichomonas vaginalis. Most people infected with Trichomonas vaginalis are asymptomatic. Symptomatic infections are characterized by vaginal discomfort, itching, discharge, dysuria and foul odor. Diagnosis depends on finding trophozoites in secretions of the genital tract from men or women. FIGUR E 2.1 T richomonas vag inalis is a flagellate, proto zo an parasite i n human vagina and urethra. T. vaginalis Actual specimen in simulation. Chlamydomonas Proper specimen collection and handling of vaginal samples is crucial for accurate results. The vaginal vault and walls should be swabbed using one or two swabs. If any fluid has pooled in other areas, these areas should be swabbed as well. The swabs should then be placed in a tube containing 0.5 ml saline and examined within two hours of collection. 2-2 Exercise 3. T HE WET MOUNT PREPA RATION PROC EDUR E Step 1. Each desk is provided a s imu lat ed suspension of Trichomonas (the specimen contains Chlamydomonas, a genus of unicellular photosynthetic flagellates -a green algae) and a sterile individual wrapped swab. Vigorously mix the swab in and out of the saline making sure to collect all the material adhering to the side of the tube. Step 2. Remove the swab from the saline and depress onto a clean, dry microscope slide expressing a small amount of fluid. Step 3. Coverslip the sample and examine under a microscope beginning with the 10X objective. The instructor will demonstrate this procedure. RESULTS Chlamydomonas reinhardi Draw your observations of motility, record the magnification and illustrate the presence of algal flagella. BACTER IAL S HAPES Figur e 2. 2 shows four of the common and characteristic shapes of bacteria. 1. Cocc i. (coccus, singular) Your example today will be Micrococcus luteus, which forms tetrads due to regular alternation of the plane of cell division –tetrads are boxes of 4 cells held together. Micrococcus species are commonly associated with the skin. Staphylococcus species also are common on the skin, and S. aureus causes boils or, occasionally, more serious infections. Streptococcus species also are common but typically form chains of cells because they divide in a single plane. Streptococcus mutans commonly grows on tooth enamel and contributes to tooth decay (de nta l car ie s ). 2-3 2. Rod s (bac illu s s hape ). Bacillus subtilis, from laboratory exercise 2, is a common inhabitant of soil but also found on many food products. B. cereus causes a relatively mild food intoxication, especially on reheated fried rice in Asian food outlets. The spores can resist destruction during cooking and can then germinate if the cooked food is not refrigerated. Two toxins are formed: one is heat-stable and is produced during sporulation causing vomiting; the other is heat-labile and is produced during exponential growth causing diarrhea. 3. Vibr io. The best-known species of the Genus Vibrio is V. cholera, which causes cholera, a severe diarrheal disease resulting from a toxin produced by bacterial growth in the gut. 4. Sp ira l. Your example today will be Rhodospirillum rubrum. Typically, these bacteria grow in shallow a naerob ic organic-rich pools, obtaining energy from light reactions but using organic substances such as acetate for cellular synthesis. [Organisms of this type are termed photo he terotrop h s]. Rhodospirillum swims in a corkscrew manner, by means of its polar flagella. Other spiral-shaped bacteria include the s piroc he te s, such as Treponema pallidum which causes syphilis ( Labora tory e xerc is e 33). However spirochetes have a different type of motility from that of the common spiral bacteria. FIGUR E 2.2 B ACTER IAL S HAP ES Bacillus, Rod shapes. Coccus, round shapes. tetrads staphylococcus streptococcus Spiral and Vibrio. Exercise 4 T HE S IMPLE STAIN Bacteria jitter about in fluid suspensions with Brownian movement or true motility; they are difficult to visualize sharply. We can see their shapes and appreciate their activity under a cover glass but it is difficult to form a complete idea of their morphology. An important part of the problem is the minute size of bacteria. Because bacteria are so small and with little substance they tend to be transparent even when magnified in subdued light. In order to visualize bacteria we must stop their motion and tag their structures with something to make them more visible to the human eye. Many sophisticated ways of doing this are known but the simplest is to smear out a suspension of cells, fix them to a slide and stain them with a visible dye. 2-4 The best bacterial stains are aniline dyes, which are synthetic organic compounds made from coal-tar products. When they are used directly on fixed bacterial smears, the contours of bacterial bodies are clearly seen. These dyes are either acidic, basic, or neutral in reactivity. Acidic or basic stains are used primarily in bacteriologic work. The free ions of acidic dyes are anions (negatively charged) that combine with an acid in the stained material to form a salt. Bacterial cells are rich in ribonucleic acid and therefore stain very well with basic dyes. A neutral stain is created by combining acidic and basic dyes. They are most useful for staining complex cells of higher forms because they permit differentiation of interior structures, some of which are basic, some acidic. Cells and structures that stain with basic dyes are said to be basophilic. Those that stain with acid dyes are termed acidophilic. CULTURE Binomial name - Genus species Rhodospirillum rubrum: Spiral shape bacteria. Bacillus subtilis: Rod shape bacteria, arranged in chains. Escherichia coli: Rod shape bacteria, arranged in single cells. Micrococcus luteus: (round) coccus shape bacteria, arranged in clusters and tetrads. PROC EDUR E The basic dyes you will be using in this exercise are crys ta l v iolet, me thy le ne blu e, car b ol fuch sin or safr an in. Step 1. CLE ANI NG TH E MI CR OS COP E S LI DE. You can save time and make better observations if you first prepare a clean slide. Using a bench towel, wipe the slide with a drop of 95% ethanol. Handle the clean slide only by the edges or with your laboratory forceps. Pass the slide through the flame of your Bunsen burner and place it on your desktop. Step 2. MAKI NG A H E AT FI X ED S ME AR. 2a. Label 3 circles (M.l – B.s. – R.r.) on the bottom of your slide with a marker. If you choose to use Bacillus subtilis (B.s.) then your bench partner should use the other rod shape bacteria: Escherichia coli (E.c.). Then place the slide right side up on your benchtop. *Label the bottom of your microscope slide. 2-5 2b. Place one drop of tap water in the center of each circle. 2c. Using your loop, aseptically retrieve cells from the stock cultures one at a time and spread them in their drops of water to the size of a dime. BE SURE YOU FLAME THE LOOP BEFORE AND AFTER EACH TRANSFER. 2d. Allo w th e s mear to a ir dry! . After drying you should be able to see a thin white film. 2e. Heat-fix the cells to the slide by passing it through the Bunsen flame three times. This will inactivate bacterial enzymes and stick the cells to the glass slide. Step 3. S TAI NING TH E SLID E 3a. Place the slide on your staining rack and flood the smear with a basic (cationic) stain. 3b. After 1 minute remove the stain and wash gently with water. 3c. Drain off excess water, blot dry with paper towel, and let the slide air dry. Step 4. OBS ER VATI ON Exa min e the s ta in ed s me ar with low -po w er 100 X, t hen move to o il- immer s ion 1000 X. Record your observations at 1000X total magnification. You may save the slide in your slide box for further study or discard the stained smear in the sharps container. Do not wipe the oil from the slide! RESUTS TABLE 2. 1 AS SI G NE D MI CR OOR G ANIS M SI MP LE STAI NI NG R ES ULTS DI AG R AM: MORP H OLOG Y AND CE LL GR OUPI NG 1000 X Micrococcus luteus Bacillus subtilis Escherichia coli Rhodospirillum rubrum Co lor p lat es 2.1 ,2.2, 2.3 2-6 TERMS AND QUEST IONS F OR STUDY 1. Define acidic and basic dyes. What is the purpose of each? 2. What is Heat fixation? Why do you Heat fix a slide to be stained? 3. How does true motility differ from Browian movement? 4. What is the value of a wet-mount preparation in the clinical laboratory? 5. List three bacteria who’s name reflects their morphology. 6. List the four types of bacterial flagella arrangements. 7. Which microscope objective is most helpful when studying bacteria? Why? 8. When staining in the clinical lab, a suspension would be made with sterile saline or water. Why? 9. Describe and discuss the differences between bacterial and protozoan flagella. 10. What type of infection do you expect to see from Trichomonas vaginalis? 2-7