motility tests
advertisement
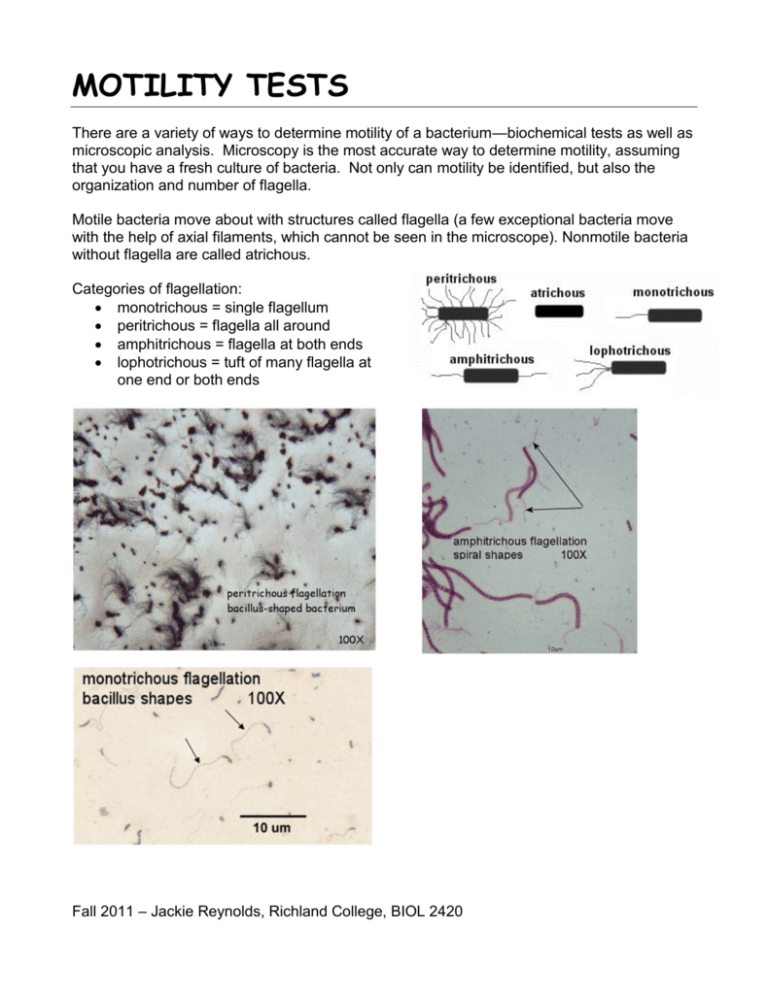
MOTILITY TESTS There are a variety of ways to determine motility of a bacterium—biochemical tests as well as microscopic analysis. Microscopy is the most accurate way to determine motility, assuming that you have a fresh culture of bacteria. Not only can motility be identified, but also the organization and number of flagella. Motile bacteria move about with structures called flagella (a few exceptional bacteria move with the help of axial filaments, which cannot be seen in the microscope). Nonmotile bacteria without flagella are called atrichous. Categories of flagellation: monotrichous = single flagellum peritrichous = flagella all around amphitrichous = flagella at both ends lophotrichous = tuft of many flagella at one end or both ends Fall 2011 – Jackie Reynolds, Richland College, BIOL 2420 Motility can be identified in a couple of different ways: - the hanging drop wet mount - motility agar media (SIM and tetrazolium motility agars used later) Looking at living bacteria are not as easy as one would think. First of all, living bacteria have no color, and they are small: therefore, they are really difficult to see, even with the oil immersion lens. Second, all bacteria have some vibrational movement, even nonmotile ones. This Brownian motion is caused by water molecules bouncing around in the solution, knocking up against each other and the microorganisms. Kinetic energy inherent to all molecules causes this kind of movement. On the other hand, those bacteria with flagella will be very apparently moving about the field of vision, although perhaps not all of the bacteria will be moving. Some cells will "run" straight across the field, others will "tumble" across the field in a slower motion. The keys to a good hanging drop slide are 1) use a small drop of bacterial suspension, but do not let it dry out, and 2) use a young culture of bacteria. Another way to determine motility---TTC motility agar with tetrazolium---will be used in lab. The tetrazolium makes the motility agar much easier to read for motility. The tetrazolium is a colorless salt which becomes red when reduced, occuring as a result of bacterial metabolism. NOTE: Strict aerobes may not grow well or at all in this medium. OBJECTIVES: Differentiate between Brownian movement and true motility. Identify flagella on bacterial cells. Differentiate among different types of flagellation. Identify motility using different methods. MATERIALS NEEDED: per table prepared flagella stains (different kinds of flagella slides in lab table boxes) (mixed flagella slides - amphitrichous, atrichous; Proteus slides - peritrichous) fresh TSB cultures of motile and nonmotile bacteria (less than 24 hours old optimally) 2 Motility agar deeps with tetrazolium dye (TTC motility agar deeps) cover slips depression microscope slides vasoline jelly in syringe 2 THE PROCEDURES: all procedures done as a table Microscopy WET MOUNT/HANGING DROP 1. Place a drop of the bacterial culture (optimally from a young broth culture) in the middle of a cover slip. Makes 2 hanging drop slides---1 for each bacterium. 2. Place a thin line of petroleum jelly at the edges of the cover slip. 3. DO NOT get petroleum jelly anywhere else. 4. Turn the depression slide upside-down (depressed area facing down) and gently touch the cover slide. The jelly holds the cover slip to the slide and also keeps the suspension from drying out. 5. Now flip the entire microscope slide/cover slip combination over. It should look like the diagram below. 6. Using brightfield microscopy, use a lower power lens to find the bacteria, then move into 100X oil immersion. PREPARED FLAGELLA STAINS These stains are bought and ready to use. Although they have cover slips, you still use oil when on 100X magnification. Be sure to remove the oil with the lens paper. You will have to move around the slide to find the best field of vision. Often, the flagella will break off and you may not see many in some areas of the slide. Biochemical media 1. Inoculate the 2 bacterial cultures into tubes of TTC motility agar with a NEEDLE, all the way to the bottom. 2. Incubate at 30 or 37 degrees C. 3. Look for the spread of the inoculum away from the inoculation line. Hold the tube up to the light and look at the stab line to determine motility. If NONMOTILE, you will see the intact straight stab line. If MOTILE, the original stab line will diffuse out into the medium as the bacteria spread throughout. BUT with the added, helpful colored dye tetrazolium which turns red as a result of the bacteria metabolizing. 3 LABORATORY REPORT SHEET QUESTIONS: 1. Make drawings from the 2 prepared slides.. peritrichous Proteus amphitrichous bacterium 2. Draw the 2 tubes of TTC media with the growth patterns of the 2 bacteria. Staph Pseudomonas 3. What designation does one used for a bacterium without flagella? 4. What is the function of tetrazolium? 5. How do you tell if the organism is motile? 6. Why is petroleum jelly used to make this slide? 7. What is the cause of Brownian movement? 8. What feature of the bacterial culture will increase the probablity of true motility? 4