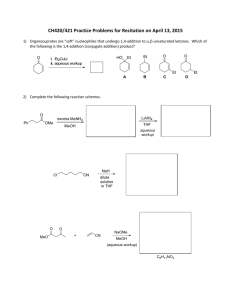
amine - mmiklavcic
advertisement

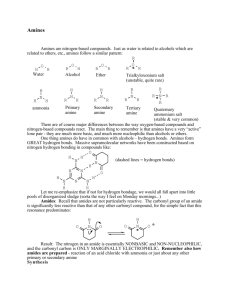
Chapter 15 Amines -derive from ammonia -one or more of the hydrogen atoms have been replaced by an organic group -pyramidal in structure -1° amine indicates 1 H replaced -2° amine indicates 2 H’s replaced -3° amine indicates 3 H’s replaced The N atom is more electronegative than the H so the N-H bond is polar Amines amines can bond to each other through hydrogen bonding and can also bond to water molecules 1° amines have higher boiling points than alkanes but lower boiling points than alcohols of similar molar mass 3° amines cannot bond to other amines because there is no H available to bond 3° amines have much lower boiling point than 1° and 2° amines of similar molar masses Amines H bonds between amines not as strong as those in alcohols because the N is not as electronegative as the O. 1° and 2° amines have a lower boiling point than alcohols All can form H bonds with water at the N Small amines are soluble in water (6 carbons or less) Solubility decreases as the carbon chain increases Amines 1. Determine the name of the parent compound (the longest continuous carbon chain containing the amine group) 2. Replace the –e ending from the alkane parent with –amine IUPAC Ex. Ethane becomes ethanamine 3. Number the parent chain so that the carbon with the amine group has the lowest number 4. Name and number any substituents and add them as prefixes Example 2-pentanamine For 2° and 3° amines, the prefix N-alkyl is added (just add the letter N) Example N,N-Dimethylpropanamine A simple benzene ring with an amine is benzenamine (aniline) See pg 499 with methyl group If other groups are attached to the N group, use the letter N- followed by the name of the group N-methyl-1-phenyl-2-propanamine Amines Common names -used for simple amines -use the common names of alkyl groups bonded to the amine and the ending –amine -list alphabetically if multiple Dimethyl amine Ethylmethylamine Amphetamines (benzedine, methadrine) -stimulates the central nervous system, elevate blood pressure and pulse rate, decrease fatigue -used to treat depression and epilepsy -found in diet pills (decrease appetite) -controlled federally because excessive use can cause mental illness and paranoia Analgesics (demerol)- pain relief Anesthetics (novocaine)- pain blocker Ephedrine -decongestant in cough syrup and nasal spray -sales of ephedrine and pseudoephedrine are now behind the counter -pharm. companies replacing these with phenylephrine Preparation of amines In a lab setting, they are prepared by reducing amides into a nitro compound. 1° amines are created by reducing a nitro compound with a reducing agent Ex. Nitrobenzene is reduced to aniline The symbol [H] is often used to show that any reduction agent can be used. 1°, 2°, 3° amines are produced when amides are reduced If the N on the amide has 2 hydrogen atoms, a 1° amine is produced If there is 1 H and 1 organic compound, a 2° amine is produced If both are organic compounds, a 3° amine is produced See page 503 for 3 different reactions Basicity When dissolved in water, an amine will accept an H+ ion and become a weak base. The lone pair from the N bonds with the H+ ion making an alkylammonium ion. Hydroxide ions are also produced. Creates a base solution. You do not need to know the reaction. You need to know why amines act as bases. -Formed from the reaction between a carboxylic acid derivative and either ammonia or an amine. -Composed of a carbonyl group (from the carboxylic acid) and an amino group (from the ammonia or amine) Amide bond- bond between the carbonyl carbon and the N that contains the amine or ammonia -Most are solid at room temp. -very high boiling points (due to strong H bonds between amides) -simple amides are soluble in water (due to strong H bonds between amides) -cannot become basic like amines (do not accept H+ in water) -there is a strong attraction between the O of the carbonyl group and the lone pair of the N which does not allow it to hold the H+ (this attraction creates a resonance hybrid) Amides Both IUPAC and Common come from the IUPAC and Common names of the carboxylic acids from which they are made. -Remove –ic acid ending from the common name or –oic acid of IUPAC name of carboxylic acid -Replace with –amide Propanamide N-Propylbutanamide See table 15.4 for IUPAC to Common comparisons Substituents on the N are placed as prefixes -indicated by N- followed by the substituent name -no spaces between prefix and amide name Barbiturates (barbital) -“downers” -used as sedatives, anticonvulsants for epileptics, brain disorders Acetaminophen -used in place of aspirin -found in Tylenol -relieves pain, reduces fever Phenacetin -pain reliever created in 1887 -banned in 1083 due to cause of kidney damage and blood disorders Preparation of Amides -prepared from carboxylic acid derivative (either acid chlorides or acid anhydrides) Recall: Acid chlorides are made from carboxylic acids that react with a reagent like PCl5 Acid chlorides react rapidly with ammonia or amines 2 moles of ammonia or amines needed in a reaction Type of reaction- Acyl group transfer reaction Acyl group- found on the acid chloride -transferred from the Cl to the N of one of the ammonia/ amine molecules -the 2nd reacts with the HCl that forms from the transfer to create ammonium chloride or alkyl-ammonium chloride See reaction page 513 The reaction between acid anhydride and 2 moles of ammonia/ amine is also an acyl group transfer reaction. See reaction page 514 Commercial Amide -aspartame -artificial sweetener -made from 2 amino acids (aspartic acid and phenylalanine) joined by an amide bond