Exam 1C
advertisement
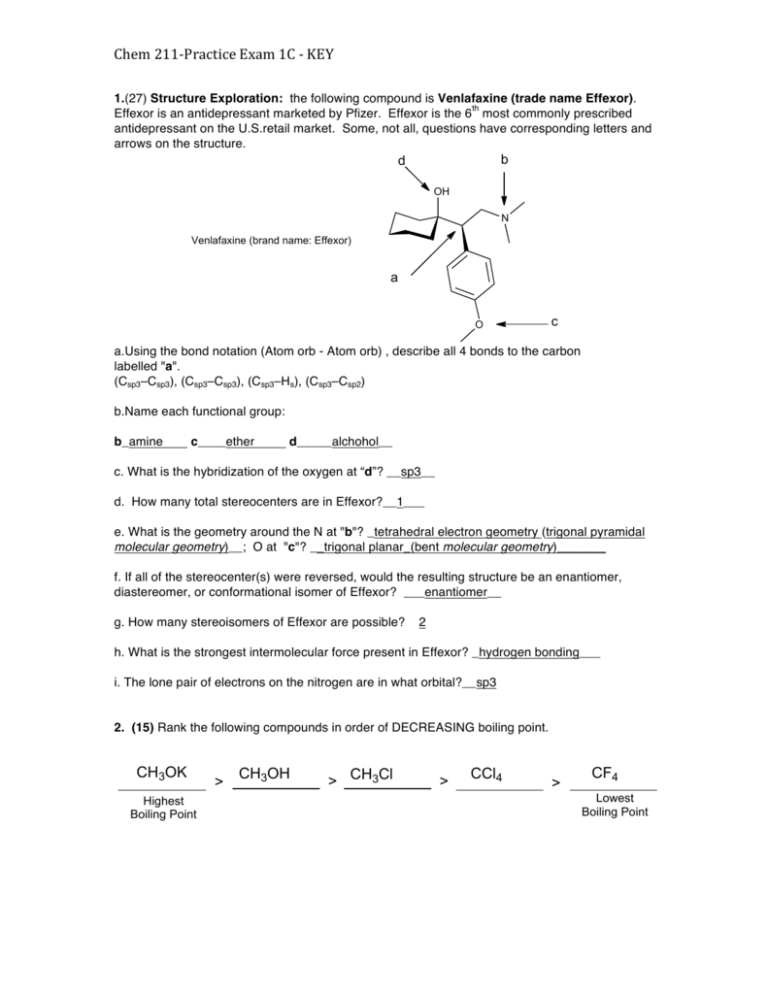
Chem 211-­‐‑Practice Exam 1C -­‐‑ KEY 1.(27) Structure Exploration: the following compound is Venlafaxine (trade name Effexor). th Effexor is an antidepressant marketed by Pfizer. Effexor is the 6 most commonly prescribed antidepressant on the U.S.retail market. Some, not all, questions have corresponding letters and arrows on the structure. b d OH N Venlafaxine (brand name: Effexor) a O c a.Using the bond notation (Atom orb - Atom orb) , describe all 4 bonds to the carbon labelled "a". (Csp3–Csp3), (Csp3–Csp3), (Csp3–Hs), (Csp3–Csp2) b.Name each functional group: b_amine c____ether d_____alchohol__ c. What is the hybridization of the oxygen at “d”? __sp3__ d. How many total stereocenters are in Effexor?__1___ e. What is the geometry around the N at "b"? _tetrahedral electron geometry (trigonal pyramidal molecular geometry)__; O at "c"? __trigonal planar_(bent molecular geometry)_______ f. If all of the stereocenter(s) were reversed, would the resulting structure be an enantiomer, diastereomer, or conformational isomer of Effexor? ___enantiomer__ g. How many stereoisomers of Effexor are possible? 2 h. What is the strongest intermolecular force present in Effexor? _hydrogen bonding___ i. The lone pair of electrons on the nitrogen are in what orbital?__sp3 2. (15) Rank the following compounds in order of DECREASING boiling point. CH3OK Highest Boiling Point > CH3OH > CH3Cl > CCl4 > CF4 Lowest Boiling Point 3.(15) In the box below, draw (using line formula) the structure of the lowest molecular mass ACYCLIC ALKANE (i.e., no rings allowed) that is chiral. No isotopes allowed (i.e., no deuterium etc)! Name the compound according to systematic IUPAC rules. 4.(15) In the box below, draw (using line formula) the structure of the lowest molecular mass CYCLIC ALKANE that is chiral. No isotopes allowed (i.e., no deuterium etc)! Name the compound according to systematic IUPAC rules. 2 5. (14) Complete the Newman projection on the right from the viewpoint of the eye for the structure in the MOST STABLE STAGGERED conformation. Label all gauche interactions with a “g”. 6. (30) RESONANCE (a) Draw TWO additional resonance structures for the CATION shown below. Show ALL curved arrows, lone pairs and nonzero formal charges. (b) Draw TWO additional resonance structures for the ANION shown below. Show ALL curved arrows, lone pairs and nonzero formal charges. (c) Draw the resonance hybrid for the structures you drew in (b). δ− δ− δ− 3 7.(15) (a) trans-1-Fluoro-3-methylcyclohexane can exist in two different chair conformations, A and B. Complete the picture of conformer B by drawing in a bond to one methyl (Me) group and one fluorine (F) atom in the appropriate positions. In the two boxes to the right, complete the corresponding Newman projections by filling in methyl (Me) groups, hydrogen (H) atoms, AND fluorine atoms (F) where appropriate. (b) (10)trans-1-Fluoro-3-methylcyclohexane is redrawn below. Assign each chiral center either an R or S configuration by writing R or S in each small box. Is this molecule the SAME as that shown in part a, or is it the ENANTIOMER, or a DIASTEREOISOMER? (c) (9)In relation to the compound shown above in part b, draw in the boxes below, (i) a diastereoisomer, (ii) a chiral constitutional isomer that still contains a cyclohexane ring, and (iii) an achiral constitutional isomer that still contains a cyclohexane ring. 4






