Nucleophilic Substitution and Elimination Reactions
advertisement

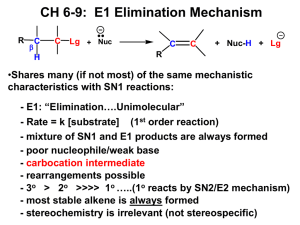
Nucleophilic Substitution and Elimination Reactions Self-Study Material printer-friendly version by Daniel Berger Nucleophilic Aliphatic Substitution R Nu + R C X R nucleophile 2 R Nu C R + R leaving group X Nucleophilic Aliphatic Substitution leaving group nucleophile R Nu + R R C X Nu R R in reverse 3 C R + X Nucleophilic Substitution in Synthesis The nucleophile attacks the alkyl halide 180o away from the halogen The SN2 Mechanism H3C HO + H C Br CH3CH2 (R)-2-bromobutane δ− HO CH3 δ− Br C H CH2CH3 Transition state with simultaneous bond breaking and bond forming 5 On the web The configuration at carbon is inverted CH3 HO C H CH2CH3 (S)-2-butanol + Br 1. H3C H C Br CH3CH2 slow ratelimiting step methanol CH3 C + Br H CH2CH3 A planar carbocation The SN1 Mechanism 2. H3C CH3 O C H H CH2CH3 CH3OH fast CH3 C H CH2CH3 CH3OH fast H C O H CH2CH3 CH3OH HOCH3 3. H3C CH3 O C H CH2CH3 (S)-2-methoxybutane On the web CH3 H3C a racemic mixture H3C CH3 H C O CH2CH3 (R)-2-methoxybutane Whether a reaction is SN1 or SN2 1. Structure of nucleophile – Also affects side reactions 2. Structure of alkyl halide substrate 3. Structure of leaving group 7 What makes a good nucleophile • Negative charge – OH- > H2O C N • Polarizability – Less electronegative – Larger • Basicity – Brønsted – Lewis 8 P O F S Cl nucleophilicity Bronsted basicity Br I Common nucleophiles and their electrophilicities 9 Side reactions in the SN1 mechanism Basicity of nucleophile NaI Br H2O Br OH NaOH Br 10 I + Whether a reaction is SN1 or SN2 1. Structure of nucleophile – Also affects side reactions 2. Structure of alkyl halide substrate 3. Structure of leaving group 11 Structure of Alkyl Halide Governed by electronic factors R SN1 R H R R H R H R H H H Increasing stability of carbocation intermediate R3CX R2CHX RCH2X CH3X (tertiary) (secondary) (primary) (methyl) Increasing ease of access to site of reaction R RC R 12 X R C RH X R C H H H X H H SN2 Governed by C X steric factors Steric Hindrance of SN2 CH3 CH3CH2Br CH3CCH2Br CH3 Rate = 1 13 Rate = 10-30 Whether a reaction is SN1 or SN2 1. Structure of nucleophile – Also affects side reactions 2. Structure of alkyl halide substrate 3. Structure of leaving group 14 Leaving Group Ability Correlates with Acid Strength reactivity as a leaving group O I > Br > Cl >> F > CH3CO > HO > CH3O > H2N strength of conjugate acid Protonated leaving groups are good, because the conjugate acids of neutral molecules are more acidic. (In other words, the leaving groups are less basic.) 15 On the web Nucleophilic substitution will not occur with a poor leaving group! good leaving groups – – – TsO ≥ X ≈ RS > HOR ≈ HNR2 16 poor leaving groups – – CN > OR > NR2 – Summary: SN1 vs. SN2 Reactions Type of Alkyl Halide SN2 SN1 methyl CH3X Favored. Does not occur because of cation instability. primary RCH2X Favored. Rarely occurs because of cation instability. secondary R2CHX Favored in aprotic solvents with good nucleophiles Favored in protic solvents with poor nucleophiles tertiary R3CX Does not occur because of sterics. Favored because of cation stability. stereocenter Inversion Racemization β-Elimination C C H2 O H OH2 C C H B C C + HB C C + HB E1 C C H X E2 C C H X B 18 On the web X C C H C C B + HB + X E2 Mechanism E1 Mechanism SN2 vs. E2 Substitution vs. elimination 22