7-1 Enthalpy and Ionic Compounds Sodium and chlorine react
advertisement

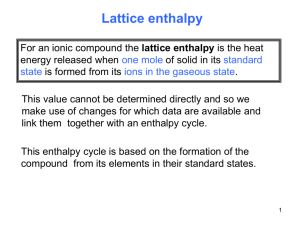
Enthalpy and Ionic Compounds Sodium and chlorine react vigorously and spontaneously to form sodium chloride. The salt can be seen as the cloud rising from the flask. 7-1 Lattice Energy: energy required to break up 1 mole of solid into gas phase ions The energy of attraction for ions to condense to form a solid can be rationalized by Colulombs Law - + Electrostatic attraction 7-2 So: Lattice Enthalpy: MX(s) M+(g) + X-(g), )H = Lattice Enthalpy The magnitudes of Lattice Enthalpies depend on the charge of the ions and their separation: The Coulomb potential energy V is given by:V = k.Q1.Q2 / r k is a constant, Q's are the charges ,r is the separation. If charges are of opposite sign, energy is released (exothermic); if they have the same sign, then energy is absorbed (endothermic). The smaller the value of r, the bigger is V. 7-3 Born Haber Cycle: just as before we can set up two equivalent paths to produce the ionic solid : example sodium chloride Na+ (g) + e- + Cl (g) enthalpy H +502 ∆H ionization energy ∆H electron affinity Na (g) + Cl (g) +121 ∆H -354 Na+ (g) + Cl- (g) at(Cl(g)) Na (g) + ½ Cl2 (g) ∆Hlattice energy +108 ∆Hat(Na(g)) Na (s) + ½ Cl2 (g) -411 ∆Hf NaCl (s) Let’s say we wish to determine this * Energies in kJmol-1 are given in green 7-4 If we know all the energies but one we can use the cycle to get it if we go completely round the cycle then we expend no energy: so if we start at * and go clockwise: -∆ ∆Hf + ∆Hat(Na(g)) + ∆H at(Cl(g)) + IE + EA + LE = 0 411 + 108 + 502 + 121 -354 - LE = 0 LE = 788 kJmol-1 7-5 Another way of looking at exactly the same problem:NaCl (s) Na(s) + 0.5Cl 2(g) )H0 = +411 kJ Na(s) Na(g) )H = +108 kJ ) H = +502 kJ Na(g) Na+(g) + e0.5Cl 2(g) Cl (g) ) H = +121 kJ Cl (g) + eCl- (g) )H = -354 kJ ADD NaCl (s) Na+(g) + Cl -(g) ) H = LE kJ Apply Hess's Law - add them all up and )H reaction has to be ZERO - why? 411 + 108 + 502 + 121 - 354 + X = 0 LE = 788kJ Lattice Enthalpy:NaCl (s) Na+(g) + Cl -(g) )H = +788 kJmol-1 it is mechanically easy to get the lattice energy: write all the values out as defined (e.g., )H (at), IE) and reverse the )Hf of the ionic solid. Then add them all up. Result is the lattice energy. 7-6 Lattice Enthalpies in kJ/mol LiF 1046 .... LiI 759 NaF 929 .... NaI 700 KF 826 .... KI 643 MgF2 2957 .... MgI2 2327 MgO 3850 .... BaO 3114 MgS 3406 .... BaS 2832 (Increased charges on ions leads to increased lattice energies.) 7-7 Example: given the following determine the electron affinity of chlorine MgCl2(s) )Hfo = –641.3 kJ/mol Mg(s) + Cl2(g) Mg(s) )H = 148 kJ/mol Mg(g) Mg(g) Mg+(g) )H = 737 kJ/mol Mg+(g) Mg2+(g) )H = 1450 kJ/mol Cl2(g) 2Cl(g) )H = 244 kJ/mol Mg2+(g) + 2Cl–(g) MgCl2(s) )H = - 2510kJmol-1 2Cl(g) 2Cl–(g) = 2EA =2(EA) So: 641.3 + 148 + 737 +1450 +244 +2EA - 2510 = 0 EA = - 355kJmol-1 7-8 Born-Haber Cycles magnesium chloride Mg2+(g) + 2e- + 2Cl enthalpy H ∆H bond energy of chlorine Mg2+(g) ∆H + 2e- + Cl2 (g) (g) 2 x ∆H Mg2+ first electron affinity (g) + 2Cl (g) second ionization energy Mg+(g) + e- + Cl2 (g) ∆H first ionization energy Mg (g) + Cl2 formation enthalpy (g) ∆H atomization Mg (s) + Cl2 ∆H ∆H lattice (g) MgCl2 (s) 7-9 Problems CaO BaO Which one has the greater lattice enthalpy? Order of Lattice Enthalpies? LiCl , NaCl , BeCl2 MgO, MgF2, MgBr2 MgCl2, BeO, BeCl2 And now we start a slightly new topic…… •2 "Spontaneous” chemical chapter 19 reactions 7-10 Introduction: Order and Disorder •2 "Spontaneous” chemical chapter 19 reactions 13.1/ Spontaneity 13.2/ Entropy: The Measure of Disorder 13.3/ Absolute Entropies 13.4/ Spontaneity and Free Energy 13.5/ Some Applications of Thermodynamics 13.6/ Bioenergetics In thermodynamics spontaneity means : will occur if left alone long enough 7-11 Schematic view of the spontaneous process for a water-and ice mixture on a table top, The energyabsorbing process, melting, is spontaneous under these conditions. Schematic view of the spontaneous process for a water-and-ice mixture in a freezer. The energyreleasing process, freezing, is spontaneous under these conditions. 7-12 It is tempting to think that chemical reactions that give off heat are spontaneous. This is close to the truth but not quite right. Recall: See page 4-10 Hot and cold packs: MgSO4(s) Mg2+(aq) + SO42-(aq) )Ho = -91.3 kJ NH4NO3(s) NH4+ (aq) + NO3-(aq) )Ho = +26.3 kJ both are spontaneous : It turns out that how disordered things become also plays a role: it will be a combination of enthalpy and entropy that will eventually tell us if a reaction will occur We need to learn about entropy: next lectures 7-13