Born-Haber Cycles & Lattice Energy Chemistry Presentation
advertisement

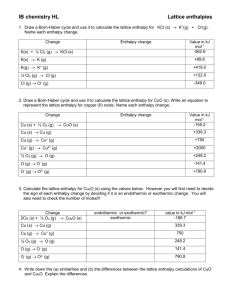
Born-Haber cycles, and lattice energy • Mr Michael (Hamzah) Allan (019 254 7120) KYUEM 5. Chemical energetics (p23) b) explain and use the term: iii) lattice energy (ΔH negative, i.e. gaseous ions to solid lattice) Definition: Lattice energy (enthalpy) is the enthalpy change when one mole of an ionic crystal (lattice) is formed from its constituent gaseous ions under standard conditions ... (Ionic) Bond forming Crystal (lattice) forms (Ionic) Bond forming DHLE = -’ve DHLE = -x kJmol-1 (Ionic) Bond --breaking-- DH = +’ve DH = +x kJmol-1 i.e. DH = - LE Examples (1/4)…. Na+(g) + Cl-(g) NaCl(s) DHLE = -781 kJmol-1 gaseous ions sign Standard state for the product Remember to state the units Examples (2/4)…. Potassium superoxide K+(g) + O2-(g) KO2(s) DHLE = -1540 kJmol-1 gaseous ions sign Standard state for the product Remember to state the units Examples (3/4) – D.I.Y. Write a thermochemcial equation for the lattice energy of barium chloride given that the 0.1 moles of the salt formed (in the appropriate manner) liberates 235.2 kJ of energy Answer = Ba2+(g) + 2 Cl-(g) BaCl2(s) DHLE = -2352 kJmol-1 Examples (4/4) Potassium peroxide 2K+(g) + O22-(g) K2O2(s) DHLE = -1980 kJmol-1 gaseous ions sign Standard state for the product Remember to state the units Points of significance about Lattice energy • It assumes a purely IONIC MODEL • Can be expressed as an equation (based on Coulombs law): 𝐿. 𝐸. = 𝑘 𝑄1 𝑄2 𝑑2 k is a constant 8.9875×109 N·m2/C2 Q1 and Q2 are the charges on the ions in coulombs (each charge = 1.602 × 10-19 C, (can include -ve for anions, + for cations) d = distance between charges in metres. 5. (e) (iv) Born-Haber cycles (P23) (including ionization energy and electron affinity) Uses of Lattice energy. • Born-Haber cycles, which shows the step-wise process of lattice formation from elements. • Born-Haber cycles have numerous uses, e.g. predicting the stability of an ionic compound. Other definitions to learn (1/3)… • Enthalpy of formation (DHf): 1 mole of a compound is formed from its elements in their standard states under standard conditions. • Enthalpy of atomization (DHat): 1 mole of gaseous atoms is formed from its element under standard conditions. Other definitions to learn (2/3)… • Ionization energy (DHIE): 1 mole of electrons is removed from 1 mole of gaseous atoms under standard conditions. NOTE: Removing e- from atoms is always an endothermic process. Energy must be supplied to overcome the attraction of the e- to the nucleus. The more e- that are removed, the more endothermic the process will be. Other definitions to learn (3/3)… • Electron affinity enthalpy (DHea): One mole of electrons is added to one mole of gaseous atoms under standard conditions. Note: The first ea’s are almost always negative. (noble gasses excepted). 2nd and later ea’s are ALWAYS positive (adding an electron to an already negative ion = repulsion to overcome) Standard conditions (1/2). • To keep all data comparable (therefore easy to use and easily transferable), measurements are usually taken under standard conditions. • 1 atmosphere (101 kPa, 760mmHg, 1 barr) • 298K (25oC – but must use K temperatures in equations) • 1 Molar solution (see electrochemistry etc) Standard conditions (2/2). • The symbol DH is accompanied with a “ ” symbol to donate standard conditions, i.e. DH If no symbol is present, assume standard conditions are used, unless stated otherwise (and mention them in definitions, e.g. “Standard enthalpy of atomization”) Born-Haber cycle for NaCl (step by step) Na+(g) + Cl (g) ½ Cl2(g) Cl(g) DHat = +122 kJ mol-1 Cl (g) Cl -(g) DHea = - 349 kJ mol-1 Na(g) + ½ Cl2(g) Na+(g) + Cl - (g) Na(g) Na+(g) DHIE = +496 kJ mol-1 Na(g) + ½ Cl2(g) Na+(g) + Cl -(g) NaCl(s) DHLE = - 787 kJ mol-1 Na(s) Na(g) DHat = +107 kJ mol-1 Datum line (zero energy line) Na(s) + ½ Cl2(g) Lattice energy <<< Elements Na(s) + ½ Cl2(g) NaCl(s) DHf = - 411 kJ mol-1 NaCl(s) product << Lattice Practice (revision guide) Born-Haber cycle for AgCl (step by step) Ag+(g) + Cl (g) ½ Cl2(g) Cl(g) DHat = +121 kJ mol-1 Cl (g) Cl -(g) DHea = - 349 kJ mol-1 Ag(g) + ½ Cl2(g) Ag+(g) + Cl - (g) Ag(g) Ag+(g) DHIE = +731 kJ mol-1 Ag(g) + ½ Cl2(g) Ag+(g) + Cl -(g) AgCl(s) DHLE = - 915 kJ mol-1 Ag(s) Ag(g) DHat = +285 kJ mol-1 Datum line (zero energy line) Ag(s) + ½ Cl2(g) Lattice energy <<< Elements Ag(s) + ½ Cl2(g) AgCl(s) DHf = - 127 kJ mol-1 AgCl(s) product << Lattice References: 1) Formation of crystal lattice from ions: http://www.everystockphoto.com/photo.php?imageId=1717296 2) NaHalide LE’s figure: http://www.chemhume.co.uk/A2CHEM/Unit%202b/9%20Lattice%20enthalpy/Ch9 Latticec.htm 3) The LiF Born Haber cycle : http://en.wikipedia.org/wiki/Born%E2%80%93Haber_cycle 4) Scaled Born-Haber diagram for NaCl and practice question: CAMBRIDGE INTERNATIONAL AS AND A LEVEL CHEMISTRY REVISION GUIDE - David Bevan. Pub: Hodder. ISBN 978 1 4441 1268 9