Mole Relationship Lab
advertisement

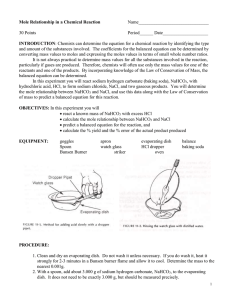
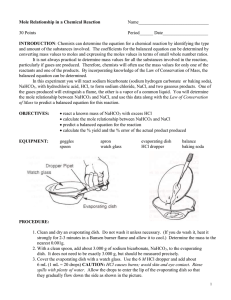
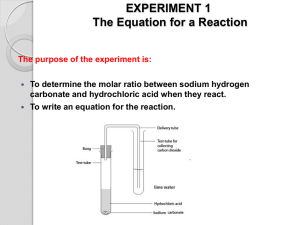
Name ____________________________ Period _________ Mole Relationship Lab INTRODUCTION: Chemists can determine the equation for a chemical reaction by identifying the type and amount of the substances involved. The coefficients for the balanced equation can be determined by converting mass values to moles and expressing the moles values in terms of small whole number ratios. It is not always practical to determine mass values for all the substances involved in the reaction, particularly if gases are produced. Therefore, chemists will often use only the mass values for one of the reactants and one of the products. By incorporating knowledge of the Law of Conservation of Mass, the balanced equation can be determined. In this experiment you will react sodium bicarbonate (baking soda), NaHCO3, with hydrochloric acid, HCl, to form sodium chloride, water vapor, and carbon dioxide gas. You will determine the mole relationship between NaHCO3 and NaCl, and use this data along with the Law of Conservation of Mass to predict a balanced equation for this reaction. Before You Begin: 1. What is the mole relationship between NaHCO3 and NaCl: HCl + NaHCO3 NaCl + H2O + CO2 2. What is the goal of this lab? 3. How will you accomplish the goal of the lab? 4. What will you have to measure accurately to accomplish that goal? PROCEDURE: 1. Clean (only if necessary) and dry your evaporating dish and watch glass. 2. Weigh the evaporating dish and watch glass together. 3. With your scoopula, add approximately 2.00 grams of baking soda, NaHCO3, to the evaporating dish. Be sure to record the actual amount used in the data table. 4. Cover the evaporating dish with the watch glass. Use the 6M HCl dropper, add HCl until the substances stop reacting. CAUTION: HCl causes burns; avoid skin and eye contact. Rinse spills with plenty of water. Allow the drops to enter the lip of the evaporating dish so that they gradually flow down the side as shown in Figure 11-1. 6. Tilt the dish from side to side to make sure the HCl has reached all of the NaHCO3, if any unreacted NaHCO3 remains, add a few more drops of HCl to complete the reaction. 7. Remove the watch glass cover with forceps and rinse the underside of the watch glass with a very small amount of distilled water from the water bottle. Be careful to wash all the material into the evaporating dish as shown in Figure 11-2. 8. Place the watch glass back on top of the evaporating dish and heat the solution to dryness using your Bunsen burner. 9. Allow the evaporating dish and watch glass to cool. Once cool, weigh the evaporating dish, watch glass and NaCl. 10. Once all of your data is collected you can clean the evaporating dish and watch glass in the sink with plenty of water. DATA TABLE: Measure to the nearest 0.01g. Mass of empty evaporating dish & watch glass Mass of evaporating dish, watch glass & NaHCO3 Mass of NaHCO3 Mass of evaporating dish, watch glass, and NaCl Mass of NaCl QUESTIONS: 1. Calculate the number of moles of NaHCO3 used in this reaction: ( 𝑔 𝑜𝑓 𝑁𝑎𝐻𝐶𝑂3 1 𝑚𝑜𝑙 𝑜𝑓 𝑔 𝑜𝑓 𝑁𝑎𝐻𝐶𝑂3 )∙( )= 1 𝑔 𝑜𝑓 𝑁𝑎𝐻𝐶𝑂3 2 2. Calculate the number of moles of NaCl produced in this reaction: ( 𝑔 𝑜𝑓 𝑁𝑎𝐶𝑙 1 𝑚𝑜𝑙 𝑜𝑓 𝑔 𝑜𝑓 𝑁𝑎𝐶𝑙 )∙( )= 1 𝑔 𝑜𝑓 𝑁𝑎𝐶𝑙 3. Write a complete balanced equation for the reaction that occurred. (see INTRO) 4. What is the mole ratio of NaHCO3 to NaCl? 5. Calculate your mole ratio of sodium chloride to baking soda by dividing the larger mole number from question 1 or to by the smaller mole number from question 1 or 2: 4. How does this compare with your mole ratio from question #3? 5. Suppose you started with 3.25 moles of sodium hydrogen carbonate, how many moles of sodium chloride would you expect to be formed? Explain. 6. Using the mass of sodium bicarbonate you started with, calculate the number of grams of sodium chloride that should have been produced. 3