Mole Relationship in a Chemical Reaction INTRODUCTION Name_______________________________
advertisement

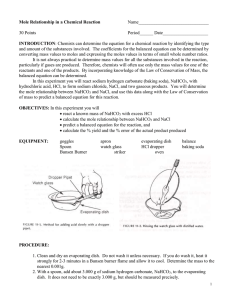
Mole Relationship in a Chemical Reaction Name_______________________________ 30 Points Period______ Date____________________ INTRODUCTION: Chemists can determine the equation for a chemical reaction by identifying the type and amount of the substances involved. The coefficients for the balanced equation can be determined by converting mass values to moles and expressing the moles values in terms of small whole number ratios. It is not always practical to determine mass values for all the substances involved in the reaction, particularly if gases are produced. Therefore, chemists will often use the mass values for only one of the reactants and one of the products. By incorporating knowledge of the Law of Conservation of Mass, the balanced equation can be determined. In this experiment you will react sodium bicarbonate (sodium hydrogen carbonate or baking soda), NaHCO3, with hydrochloric acid, HCl, to form sodium chloride, NaCl, and two gaseous products. One of the gases produced will extinguish a flame, the other is a vapor of a common liquid. You will determine the mole relationship between NaHCO3 and NaCl, and use this data along with the Law of Conservation of Mass to predict a balanced equation for this reaction. OBJECTIVES: react a known mass of NaHCO3 with excess HCl calculate the mole relationship between NaHCO3 and NaCl predict a balanced equation for the reaction calculate the % yield and the % error of the actual product produced EQUIPMENT: goggles spoon apron watch glass evaporating dish HCl dropper balance baking soda PROCEDURE: 1. Clean and dry an evaporating dish. Do not wash it unless necessary. (If you do wash it, heat it strongly for 2-3 minutes in a Bunsen burner flame and allow it to cool.) Determine the mass to the nearest 0.001g. 2. With a clean spoon, add about 3.000 g of sodium bicarbonate, NaHCO3, to the evaporating dish. It does not need to be exactly 3.000 g, but should be measured precisely. 3. Cover the evaporating dish with a watch glass. Use the 6 M HCl dropper and add about 6 mL (1 mL = 20 drops) CAUTION: HCl causes burns; avoid skin and eye contact. Rinse spills with plenty of water. Allow the drops to enter the lip of the evaporating dish so that they gradually flow down the side as shown in the picture. 1 4. Continue adding the acid slowly until the reaction has stopped. Do not add more acid than is needed, but no solid should remain. 5. Tilt the dish from side to side to make sure the HCl has reached all of the NaHCO3, if any unreacted NaHCO3 remains, add a few more drops of HCl to complete the reaction. Remove the watch glass cover with forceps and rinse the underside of the watch glass with a very small amount of distilled water from the water bottle. Be careful to wash all the material into the evaporating dish as shown in the picture. 6. Return the watch glass to your drawer, write your names on a paper towel, and place the evaporating dish on the paper towel on the tray on the front lab bench. Complete A, B, C in the data table and questions #1, 3-6. One of the gases produced will extinguish a flame, the other is a vapor of a common liquid. 7. After the product has been allowed to evaporate overnight, weigh the product to the nearest 0.001g. 8. Complete the data table and questions #1 and #2 and check your answers with your teacher. Then the contents of the evaporating dish may then be rinsed down the drain with plenty of water. Return the evaporating dishes to the front lab counter. DATA TABLE: Measure to the nearest 0.001g. A Mass of empty evaporating dish B Mass of evaporating dish and NaHCO3 C Mass of NaHCO3 D Mass of evaporating dish and NaCl E Mass of NaCl QUESTIONS: 1. Calculate the number of moles of NaHCO3 used in this reaction: 2. Calculate the number of moles of NaCl produced in this reaction: 3. Write a complete balanced equation for the reaction that occurred. (see Intro and Procedure #6) 2 4. From your balanced equation, what is the mole ratio between NaHCO3 and NaCl? _______________ How does this compare with your mole ratio from questions #1 to #2. Give a reason why they are similar or different. 5. Suppose you started with 3.25 moles of sodium bicarbonate, how many moles of sodium chloride would you expect to be formed? ____________ If you started with “X” moles of NaHCO3 how many moles of NaCl would you expect to be formed? Explain. 6. Using the mass of sodium bicarbonate, NaHCO3, calculate the number of grams of sodium chloride that should have been produced. This is the expected (experimental or theoretical) yield. 7. Calculate the % yield for NaCl produced in the experiment, by comparing it to the amount of NaCl that should have been recovered. actual % Yield x 100% expected 8. Calculate the % error for NaCl produced in the experiment, by comparing it to the amount of NaCl that should have been recovered. expected - actual % Error x 100% expected 9. Was your actual yield of salt close to your theoretical yield (was your % yield exactly 100%)? If not, list at least 2 possible sources of error. 3