Clinical significance and antimicrobial susceptibility of rapidly

Science against microbial pathogens: communicating current research and technological advances
_______________________________________________________________________________
Clinical significance and antimicrobial susceptibility of rapidly growing mycobacteria
L. García-Agudo 1 , P. García-Martos 2 .
1 Microbiology Unit, Laboratory of Clinical Analysis, Hospital General de Tomelloso, Vereda de Socuéllamos s/n, 13700
Tomelloso, Ciudad Real, Spain
2 Mycobacteria and Mycology Unit, Service of Microbiology, Hospital Universitario Puerta del Mar, Avda. Ana de Viya
21, 11009 Cádiz, Spain
Atypical mycobacteria are ubiquitous in nature and widely distributed in water, soil and animals. Although a large number of species have been identified, only a few have clinical interest in humans. The most prevalent rapidly growing mycobacteria (RGM) in human infections are Mycobacterium fortuitum group, M. chelonae group and M. abscessus ; other species are minority and only referred to opportunistic infections.
During the past three decades we have observed a notable increment of infections caused by RGM, both localized and disseminated, as well as nosocomial outbreaks of contaminated medical equipment. Pulmonary, skin and soft tissue are the most frequent locations. Other infections include keratitis, endophthalmitis, arthritis, osteomyelitis, endocarditis, meningitis, peritonitis, urinary tract infection, chronic otitis media after tympanostomy tube implantation and catheterrelated bacteremia. They are mostly due to accidental inoculation from trauma, surgery, injection or aspiration. There is no evidence of interhuman transmission.
The microbiological diagnosis of RGM infections includes direct microscopic observation of the microorganism in clinical samples and culture in selective media: Löwenstein-Jensen solid medium and Middlebrook 7H9 broth. The identification to the species level is really important to direct the antimicrobial treatment. The taxonomic identification is performed by phenotypic, biochemical and chromatographic techniques, as well as molecular biology techniques: solid-phase hybridization, nucleic acid sequencing (16S rRNA gene) or polymorphism analysis of restriction fragments of the hsp 65 gene (PRA or PCR-RFLP).
The treatment of infections caused by RGM differs from that of other mycobacteriosis like tuberculosis, owing to the variable in vitro susceptibility of this group. The RGM are resistant to conventional antituberculous drugs but can be susceptible to other broad spectrum antibiotics. The susceptibility to antibiotics varies among different species. The broth microdilution is the recommended method to determine it. Susceptibility tests offer guidance on clinical treatment.
In this chapter, we comment relevant aspects of human infections by rapidly growing mycobacteria, including biology, epidemiology, pathology, microbiological diagnosis, taxonomic identification, antimicrobial susceptibility and treatment.
Key words Mycobacterium , rapidly growing mycobacteria, atypical mycobacteria, Mycobacterium fortuitum ,
Mycobacterium chelonae , Mycobacterium abscessus , antimicrobial agents, antituberculous drugs.
1. Introduction
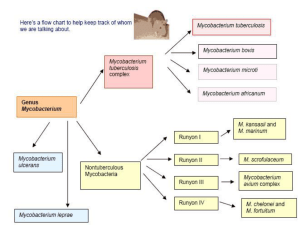
The genus Mycobacterium is included in the family Mycobacteriaceae and the order Actinomycetales , phenotypically most closely related to members of Nocardia , Rhodococcus and Corynebacterium . Mycobacteria appear as straight or slightly curved rods between 0.2-0.6 µm wide by 1.0-10 µm long, Gram positive, non-motile, non-spore forming, obligate aerobes. Their cell wall has a high lipid content, responsible for acid resistance in Ziehl-Neelsen stain. They are intracellular organisms resistant to the environmental conditions. Many Mycobacterium species readily adapt to growth on very simple substrates but some species can be very difficult to culture. Optimum growth temperatures widely vary according to the species and range from 25°C to over 50°C.
A natural division occurs between slow growth (>7 days) and rapid growth (<7 days) species. Mycobacteria that form clearly visible colonies to the naked eye within seven days on subculture are termed rapid growers, while those requiring longer periods are termed slow growers. Some mycobacteria produce deep yellow to orange colonies when grown in the presence of either the light or dark (schotochromogens), others produce non-pigmented colonies when grown in the dark and pigmented colonies only after photoactivation (photochromogens), and others are non-pigmented in the light and dark or have a pale yellow, buff or tan pigment that does not intensify after light exposure (nonchromogens) [1,2]. From a microbiological point of view mycobacteria are classified into six major groups proposed by
Runyon, according to the growth rate and pigmentation to growth in Löwenstein Jensen solid medium: I. Slow growth photocromogens; II. Slow growth schotocromogens; III. Slow growth non-chromogens; IV. Rapid growth photocromogens; V. Rapid growth schotocromogens; VI. Rapid growth non-chromogens.
Atypical or environmental mycobacteria are known as all those who are not part of Mycobacterium tuberculosis and
Mycobacterium leprae . They have an identical morphology but show some differences in their growth in culture, lipid constituents and their biochemical, antigenic and genetic profiles. Most are environmental mycobacteria that seldom
©FORMATEX 2011 363
Science against microbial pathogens: communicating current research and technological advances
______________________________________________________________________________ cause disease in humans. Nearly a hundred of rapidly-growing mycobacteria (RGM) have been identified. Although the general recognition of RGM can be made with confidence, further species identification becomes difficult, particularly by biochemical methods, as with many nontuberculous slow growers. Although many ones have been known for some time, most of them have been described in the recent years by molecular biology methods [3,4]. Moreover some have been reclassified as species, particularly members of the Mycobacterium fortuitum complex. Mycobacterium fortuitum group includes M. fortuitum, M. peregrinum, M. mucogenicum, M. senegalese, M. mageritense and the several recently described species M. septicum, M. alvei, M. houstonense, M. boenickei, M. conceptionense, M. porcinum, M. neworleansense y M. brisbanense.
Mycobacterium chelonae group includes M. chelonae and M. abscessus .
Mycobacterium mucogenicum group includes M. mucogenicum, M. aubagnense and M. phocaicum. Mycobacterium smegmatis group includes M.smegmatis, M. goodii and M. wolinskyi [1,2,5,6].
Table 1 shows the rapidly growing mycobacteria species identified until recent years, classified according to the production of pigment.
Table 1.
Rapidly growing mycobacteria species classified according to the production of pigment.
Non-chromogens rapidly growing mycobacteria
M. abscessus
M. agri
M. alvei
M. aubagnense
M. barrassiae
M. bonickei
M. bolletii
M. brisbanense
M. brumae
M. canariasense
M. chelonae
M. chitae
M. conceptionense
M. confluentis
M. elephantis
M. fallax
M. fortuitum
M. friedmanii
M. goodii
M. hackensackense
M. houstonense
M. immunogenum
M. mageritense
M. massiliense
M. moriokaiense
M. mucogenicum
M. neworleansense
M. peregrinum
M. phocaicum
M. porcinum
M. pulveris
M. salmoniphilum
M. senegalense
M. septicum
M. sphagni
M. wolynski
Photochromogens rapidly growing mycobacteria
M. marinum
M. novocastrense
Schotochromogens rapidly growing mycobacteria
Pik-red pigment
M. engbaecki
M. rhodochrous
Yellow-orange pigment
M. acapulcense
M. aichiense
M. aurum
M. chlorophenolicum
M. chubuense
M. cosmeticum
M. duvalii
M. flavescens*
M. frederiksbergense
M. gadium
M. gilvum
M. hassiacum
M. hodleri
M. holsaticum
M. komossense
M. lacticola
M. madagascariense
M. monacense
M. murale
M. neoaurum
M. obuense
M. poriferae
M. rhodesiae
M. tokaiense
M. vanbaalenii
Irregular pigment
M. austroafricanum
M. confluentis
M. diernhoferi
M. parafortuitum
M. phlei
M. smegmatis
M. thermoresistibile
M. thamnopheos
M. vaccae
364 ©FORMATEX 2011
Science against microbial pathogens: communicating current research and technological advances
_______________________________________________________________________________
Atypical mycobacteria are ubiquitous in nature and widely distributed in water, soil and animals. Water and soil are the main sources of infection in human infections. RGM can also be found in dust, rocks and bioaerosols. These organisms have been increasingly identified from environments with harsh conditions (low nutrients, low pH, and extreme temperatures) [7]. Biofilm formation is a successful survival strategy for these very hydrophobic organisms. In fact, their presence in early biofilms in water pipes may make them real biofilm “pioneers”. Rapidly growing mycobacteria are difficult to eradicate with common decontamination practices and are relatively resistant to standard disinfectants such as chlorine, organomercurials, and alkaline glutaraldehydes [8]. Dispersal from biofilms may be a mechanism of shedding from a device or water pipe to infect the patient. In piped water systems, multiple mycobacterial species have been described. In hot water systems, several thermophilic mycobacteria can survive and have been reported to cause outbreaks or pseudo-outbreaks [9]. In cold water systems, Mycobacterium fortuitum, M. chelonae, M. abscessus and Mycobacterium mucogenicum have been found. Because both cold and hot water temperatures exist in nosocomial settings, it is not surprising to see an array of species responsible for infection.
2. Infections caused by rapidly growing mycobacteria
Rapidly-growing mycobacteria have emerged as significant human pathogens, causing various infections in healthy and immunocompromised hosts. The first cases of disease caused by atypical or environmental mycobacteria were described in the decade of the fifties. The set of these diseases is called mycobacteriosis. For many years these infections were occasional, but in the last 15 years they have become relatively common. The majority of infections are due to accidental inoculation from trauma, surgery, injection or aspiration, however there is no evidence of transmission from person to person. Noteworthy it is the fact that most infections occur in patients with any underlying disease or risk factor added. The development of modern methods of microbiological diagnosis has allowed the isolation and identification of a big number of new species, some of them difficult to grow and complex to characterize, which are related to nosocomial and serious infections in immunocompromised patients with malignancies [3,4,7,10]. The new liquid culture media using Middlebrook broth to detect the growth in automated reading systems, the high pressure liquid chromatography (HPLC), which determines the composition of mycolic acids, and the amplification technology to analyze genetic variability restriction profiles of the amplified hsp-65 gene by PCR-RFLP (Restriction Fragment
Length Polymorphism) or the sequencing of 16S ribosomal RNA, have significantly contributed to the identification of the new species [1,2,7,11,12].
During the past three decades there has been a significant increase in post-traumatic and post-operative infections due to these organisms, and in recent years RGM have been frequently associated with localized and disseminated infections, including outbreaks of infection due to contamination of medical equipment. However, to consider the RGM as pathogenic, it is necessary to analyze the clinical and laboratory data, the source of isolation and the crop characteristics, to demonstrate the presence of the isolated species in other patient samples or repeated crops of the same sample, and to observe the patient's evolution after specific treatment.
Rapidly growing mycobacterial infections have been increasingly reported within a medical or paramedical scenario associated to:
Catheters : indwelling venous access catheters, vascular shunts, epidural catheters or Tenckhoff catheters, in relation to immunosuppression, long duration of the catheter placement and prior antimicrobial therapy. They are cause of bacteraemia, catheter tunnel infection, meningitis or peritonitis [2,10,13-15].
Dialysis procedure , both intravascular and peritoneal mechanisms of renal replacement therapy, in relation to contamination of the aqueous solutions used to sterilize the reusable dialysis filters, the catheter insertion site, the tunneling tract and/or the peritoneum. It leads to bacteraemia or peritonitis [16,17].
Injections : contaminated solutions of local anesthetic agents, steroids dispensed in multiuse vials, adrenal cortex injections in individuals following naturopathic or weight loss programs, reused needles or rinsed in tap water, etc. resulting in abscesses formation.
Surgical supplies : contaminated surgical instruments, implants, prosthetic valves, tympanostomy tubes, suture material or solutions [2,7,18].
Surgery : laser vision-correction surgery, facial procedures, abdominoplasty, liposuction, breast reduction or augmentation, mammoplasty and nipple piercing, as causes of post-surgical infections. Contributing factors may include the use of alternative medicine providers and the performance of these practices in freestanding surgical centers not routinely monitored by infection-control committees or equivalent oversight bodies [19-
21].
Pulmonary disease may be associated with structural lung disease and impaired clearance of the organisms, as it is seen in patients with cystic fibrosis, bronchiectasis, and chronic vomiting. Clinically the infection can range from an asymptomatic, indolent disease with minimal clinical symptoms to severe bronchiectasis and cavitary lung disease [22].
Hypersensitivity pneumonitis is mostly seen in people working with metal-working fluids that are contaminated with mycobacteria, although it may also occur after contact with indoor hot tubs, spas and swimming [23-25].
Disseminated infections are characterized by the presence of non-contiguous multiple nodular lesions, usually in the extremities but rarely affecting organs, and sometimes accompanied by fever. The finding of a disseminated disease
©FORMATEX 2011 365
Science against microbial pathogens: communicating current research and technological advances
______________________________________________________________________________ should alert the clinician to an immunocompromising condition, such as malignancy, transplantation, HIV infection, cell-mediated immunity defects, lymphoma, leukemia, corticosteroid therapy, chronic renal failure, collagen vascular disease or defects in cytokine pathways [26].
Other infections caused by RGM include keratitis, endophthalmitis, arthritis, osteomyelitis, endocarditis, meningitis, lymphadenitis, peritonitis, urinary tract infection, hepatitis, chronic otitis media after tympanostomy tube implantation, mastoiditis, pacemaker leads infection, tenosynovitis, pleural infection and furunculosis after whirlpool footbaths [10].
3. Rapidly growing mycobacteria of clinical interest
As a result of the widespread use of 16S ribosomal RNA gene sequencing, about fifty RGM species have been described but only a few have clinical significance. From a clinical point of view RGM are mainly opportunistic pathogens. The species most commonly recovered from patients belong to the Mycobacterium fortuitum complex,
Mycobacterium chelonae, Mycobacterium abscessus , Mycobacterium mucogenicum , and Mycobacterium smegmatis , reported almost everywhere worldwide. Other RGM species may occasionally cause disease in humans.
Table 2 shows rapidly growing mycobacteria according to the production of pigment and its importance in human infections.
Table 2.
Rapidly growing mycobacteria: classification according to the production of pigment and its importance in human infections.
Group Opportunistic pathogens
Casual pathogens
Photochromogens
Schotochromogens
M. marinum* M. novocastrense
M. aurum
M. cosmeticum
M. flavescens*
M. neoaurum
M. phlei
M. smegmatis
M. thermoresistibile
M. vaccae
Non-chromogens M. abscessus
M. chelonae
M. fortuitum
M. mucogenicum
M. bolletii
M. bonickei
M. brisbanense
M. brumae
M. conceptionense
M. goodii
M. houstonense
M. immunogenum
M. mageritense
M. massiliense
M. neworleansense
M. peregrinum
M. porcinum
M. senegalense
M. septicum
M. wolinskyi
Rarely pathogens
M. austroafricanum
M. confluentis
M. frederiksbergense
M. hassiacum
M. hodleri
M. holsaticum
M. lacticola
M. monacense
M. rhodesiae
M. rhodochrous
M. tokaiense
M. alvei
M. aubagnense
M. barrassiae
M. canariasense
M. elephantis
M. hackensackense
M. phocaicum
Usually nonpathogens
M. acapulcense
M. aichiense
M. chlorophenolicum
M. chubuense
M. diernhoferi
M. duvalii
M. engbaecki
M. gadium
M. gilvum
M. komossense
M. madagascariense
M. murale
M. obuense
M. poriferae
M. parafortuitum
M. thamnopheos
M. vanbaalenii
M. agri
M. chitae
M. confluentis
M. fallax
M. friedmanii
M. moriokaiense
M. pulveris *
M. salmoniphilum
M. sphagni
* It also may be slow growth.
366 ©FORMATEX 2011
Science against microbial pathogens: communicating current research and technological advances
_______________________________________________________________________________
The RGM species which are considered opportunistic pathogens are the following ones:
Mycobacterium marinum , which typically grows optimally at 30°C, often within the range of time considered to define the mycobacterial species as rapid growth, but when the culture is incubated at 37°C it appears as a slow-growing species. This makes the species can be included both among the rapid and the slow-growing mycobacteria. Mycobacterium marinum causes swimming pool or aquarium granuloma, in patients with epidemiological history of contact with contaminated water in swimming pools or aquariums, where the mycobacterium enters the skin through continuity’s solutions. Once in the body, it causes an indolent granulomatous lesion that may end fistulized. Much more rarely, M. marinum associates to other medical conditions, such as bone and joint infections, tenosynovitis, arthritis and osteomyelitis [27-31]. Disseminated infection is exceptional [32].
Mycobacterium is an ubiquitous organism that can be isolated from different aquatic habitats and soil and may contaminate water supplies, reagents and washing solutions for hospitals. This is due to its ability to survive in the absence of nutrients and within a wide range of temperatures. It often causes pulmonary infection, chronic lung disease, endocarditis, chronic otitis media, disease after laser in situ keratomileusis surgery, surgical wound, post-injection, catheter and hemodialysis-related infections, as well as disseminated
infections in immunosuppressed patients [25,33-41].
Mycobacterium is one of the most pathogenic RGM which shows greater resistance to antibiotics.
The most common clinical picture is the skin disease, sometimes disseminated, generally in patients under immunosuppressive therapy for solid organ transplant, rheumatoid arthritis or other autoimmune process. It can also produce traumatic localized infection (cellulitis, abscesses and osteomyelitis), surgical wound infection, post-injection disease and that related to intravascular catheters [26,34,42-46].
Mycobacterium fortuitum has been found in sternal wound infections, post-injection abscess disease, plastic surgery-related disease, traumatic osteomyelitis, cellulitis, mastitis, peritonitis and, among others, intravenous
catheter-related infection. Otherwise pulmonary and disseminated infections are rare [26,34-36,47-53].
Mycobacterium owes its name to the mucoid appearance of its colonies. Although it has been frequently recovered in drinking water and spitting, being a simple contaminant, the mycobacterium’s pathogenic capacity has been demonstrated in several nosocomial outbreaks in patients under dialysis, intravenous catheter-related infections, central nervous system diseases, respiratory infections, skin and soft tissue infections, bacteremia and disseminated infections [19,36,54-57].
The implication as pathogens of other RGM is reduced to sporadic isolated cases:
Chromogenic species
Mycobacterium has been described as the causative agent of catheter-related bacteremia in some
immunocompromised patients, and bilateral pneumonia in a patient receiving infliximab therapy [58-60].
Mycobacterium has been recovered from a footbath drain and a granulomatous subdermal lesion in a patient undergoing mesotherapy [61].
Mycobacterium , classified as both rapid and slow-growing because of its intermediate growth rate, has been held responsible for pulmonary infection, keratitis, osteomyelitis, gluteal abscess and disseminated post-injection infection [62-64].
Mycobacterium neoaurum has been reported in catheter-related bacteremia, endocarditis and
meningoencephalitis [65-70].
Mycobacterium has been associated to peritonitis as a complication of chronic peritoneal dialysis, septic arthritis, infection of the foot and cardiac device-related infections [71-74].
Mycobacterium has been described as the causative agent of pulmonary disease, catheter-related bacteremia, skin and soft tissue infection, endocarditis, arthritis, osteomyelitis, lymphadenitis and disseminated infection [75-81].
Mycobacterium is a species that is characterized by its ability to grow at 52°C and has been
described as a cause of lung infection and skin and soft tissue infection after surgery [82-85]. vaccae has been reported to cause skin and lung infection [86,87].
Non-chromogenic species
Mycobacterium has been recovered from infections after laparoscopic and cosmetic surgery, respiratory and disseminated infections [88-91].
Mycobacterium have been isolated in osteomyelitis, surgical and traumatic wound infections [6].
Mycobacterium has been held responsible for surgical and traumatic wound infections,
osteomyelitis and catheter-related bacteremia [6,36]. brumae has been associated to catheter-related bacteremia [36,92].
©FORMATEX 2011 367
Science against microbial pathogens: communicating current research and technological advances
______________________________________________________________________________
Mycobacterium has been involved in infections after face rejuvenation with fat grafting and
breast implant surgery, subcutaneous abscess and post-traumatic osteitis [93-96].
Mycobacterium causes cellulitis, bursitis, osteomyelitis, post-traumatic wound infection, surgical infection and chronic lung disease [5,97-99].
Mycobacterium has been reported in surgical and traumatic wound infections and osteomyelitis
[6].
Mycobacterium immunogenum has been associated to catheter-related infection, skin infection, disseminated infection, keratitis, respiratory infection and arthritis [23,100-103].
Mycobacterium is responsible for surgical and catheter infections and severe sinusitis [104].
Mycobacterium has been found in infections after laparoscopic and cosmetic surgery,, cutaneous
infection in a "hot spa" and pneumonia [88,105-107]. neworleansense has been related to surgical and traumatic wound infections and osteomyelitis
[6]. peregrinum has been reported in infections of lung and sternal wounds, cutaneous disease,
infections related to surgical site and catheter-related infections [22,108-112].
Mycobacterium has been involved in peritonitis in a patient under continuous ambulatory peritoneal dialysis, osteomyelitis and catheter-associated bacteremia [6,36,113,114].
Mycobacterium has been described in catheter-associated bacteremia [36,115].
Mycobacterium has been isolated in catheter-related bacteremia and pneumonia [36,116,117].
Mycobacterium has been associated to cellulitis, osteomyelitis, and surgical wound infection following facial plastic surgery [5].
Other species has been isolated from human samples:
Chromogenic species
Mycobacterium from a biopsy of a cutaneous granulomatous lesion, and expectorations
[118,119].
Mycobacterium
Mycobacterium
Mycobacterium
from sputum [121].
frederiksbergense from infection after mesotherapy and soft tissue infection [46,122].
Mycobacterium
Mycobacterium
from urine [123].
hodleri from an opportunistic infection in the course of a rheumatoid arthritis [120].
Mycobacterium
Mycobacterium
from sputum, urine, and gastric fluid [124].
lacticola
Mycobacterium
Mycobacterium
from infection of the hand and pulmonary tumor [126,127].
rhodesiae from peritonitis in continuous ambulatory peritoneal dialysis [128].
Mycobacterium .
Mycobacterium from caseous necrotic granuloma in the pituitary stalk [131].
Non-chromogenic species
Mycobacterium from sputum [132].
Mycobacterium from respiratory infection and sepsis [57].
Mycobacterium from a patient with chronic pneumonia [133].
Mycobacterium from the blood of a patient with febrile syndrome [134].
Mycobacterium from the sputum and granulomatous tissue of an axillary lymph node [135].
Mycobacterium from a patient with sepsis [136].
Mycobacterium from catheter-associated bacteremia [57].
4. Microbiological diagnosis of infections by rapidly growing mycobacteria
The microbiological diagnosis of rapidly growing mycobacteria infections includes direct microscopic observation of the microorganism in the samples, culture in selective media and identification of the isolated species by phenotypic, biochemical, molecular and chromatographic techniques [137]. The finding of acid-fast bacilli (AFB) in stained smears by the Ziehl-Neelsen or auramine techniques examined under a microscope is the first evidence of the presence of mycobacteria in a clinical specimen. Accompanied by clinical data it can help to establish the presumptive diagnosis of mycobacteriosis, but we must bear in mind that all mycobacteria share the acid-resistance and microscopic morphological characteristics what does not differenciate between species. Crop and identification are necessary requisites for the diagnosis.
368 ©FORMATEX 2011
Science against microbial pathogens: communicating current research and technological advances
_______________________________________________________________________________
Estas micobacterias se aislaron tras cultivo en medio sólido de Lowenstein-Jensen y en medio líquido Middlebrook
7H9 procesado en el sistema automatizado Bactec MGIT 960 (Becton-Dickinson, Reino Unido)La identificación de las cepas se realizó mediante técnicas fenotípicas (temperatura de crecimiento, velocidad de crecimiento en medio sólido y formación de pigmento), técnicas bioquímicas (reducción de nitratos, producción de arilsulfatasa y ureasa, hidrólisis del tween 80, crecimiento en presencia de ClNa al 5% y en agar de Mac Conkey sin cristal violeta y utilización de manitol, inositol y sorbitol), y el método molecular INNO-LiPA Mycobacteria v2 (Innogenetics, BélgiSamples can be grown in solid and liquid media. The traditional method for culturing mycobacteria includes inoculation of egg-based medium such as Lowenstein-Jensen, but also media without egg, such as Middlebrook 7H10 and 7H11 are used. At present, it is recommended a primary culture of all samples in liquid media (Middlebrook 7H9 medium supplemented with enrichment substrates and inhibitors for bacteria and fungi) and incubation and reading in automated systems such as
Bactec MGIT 960, MB/BacT Alert 3D or ESP Culture System II.
There are species of RGM with growth special features. The isolation of M. haemophilum requires culture media containing hemin (medium with 1% ferric citrate ammonium), and grows best at incubation temperature of 30-32°C.
We must also account for species that need lower or higher growth temperatures, as M. marinum (30ºC) and M. thermorresistibile (52ºC).
The taxonomic identification is performed by phenotypic techniques (growth temperature, growth rate on solid medium and pigment formation), biochemical techniques (reduction of nitrate, production of arylsulfatase and urease, tween 80 hydrolysis, growth in the presence of NaCl 5% and MacConkey agar without crystal violet and the use of mannitol, inositol and sorbitol), chromatographic techniques, and molecular biology techniques: solid-phase hybridization (INNO-LiPA Mycobacteria, GenoType Mykobacterien), sequencing of the 16S ribosomal RNA gene, polymorphism analysis of restriction fragments of the hsp 65 gene (PRA or PCR-RFLP). Molecular biology techniques recognize either lipopolysaccharides, specific proteins or certain sequences of DNA, allowing an increased sensitivity compared to that of conventional tests used in the microbiological diagnosis. They enable the identification of microorganisms difficult to culture, dangerous to manipulate or impossible to identify by conventional methods and the detection of microorganisms in dormant state, as microbial genetic information does not depend on the viability of microorganisms. These methods permit the direct analysis of genes in the DNA or, alternatively, gene transcription in the form of RNA, which is also useful for the direct detection of microorganisms in clinical samples, substituting microscopy, little specific and sensitive, and overcoming the slowness or failure of the crop. They are fast and sensitive methods which allow preliminary recognition of new mycobacterial taxa and have a high biological safety, because the stability of DNA provides a safe handling and storage, apart from maintaining a proper cost-benefit ratio. A major disadvantage they present, apart from potential contamination and direct use limitations on clinical samples, is that there is little marketing of many of these techniques and some, such as sequencing, require high initial investment. But several of them may be perfectly set in laboratories without requiring a large expenditure with little maintenance and good performance [138-147].
5. Antimicrobial susceptibility of rapidly growing mycobacteria
The management of rapidly growing mycobacteria infections comprises medical treatment with various antimicrobial agents based on susceptibility patterns, sometimes besides surgical treatment as in the case of lymphadenitis and skin and soft tissue infections. The treatment of infections caused by RGM differs from that of other mycobacteriosis like tuberculosis, owing to the variable in vitro susceptibility of this group. RGM are resistant to conventional antituberculous drugs but can be susceptible to other broad spectrum antibiotics. Moreover, different species show a great variability in their response to commonly used antimicrobials in clinical practice. This situation motivates to correctly identify each clinical isolate and study its susceptibility pattern. Series studies with large numbers of strains can guide the empiric therapy, given the global knowledge of the susceptibility of various species of mycobacteria in specific geographic areas. However, it is recommended an individual study of each isolate, because the differences in the results of susceptibility may have a considerable significance for treatment.
Although there is no unanimous agreement on the indications to carry out susceptibility studies in mycobacteria, some recommended scenarios are:
Clinically significant isolates in patients previously treated with macrolides
Bacteremia in patients who are on prophylaxis with macrolides
Isolates in patients who relapse during treatment with macrolides
Initial isolates in patients with firmly diagnosed disseminated or respiratory disease
In addition, susceptibility studies should be repeated in patients with chronic pulmonary or disseminated disease at three and six months respectively, whether they show no improvement or a clinical deterioration and cultures remain positive.
In order to optimize susceptibility testing and facilitate the interpretation of susceptibility results, the former National
Committee for Clinical Laboratory Standards (NCCLS), now known as Clinical and Laboratory Standards Institute
(CLSI), recently published guidelines and recommendations for testing nontuberculous mycobacteria (CLSI, M24-A,
2003) [148,149]. It contains revised guidelines for the testing of M. tuberculosis complex and newly proposed
©FORMATEX 2011 369
Science against microbial pathogens: communicating current research and technological advances
______________________________________________________________________________ guidelines for the testing of some nontuberculous mycobacteria, including some rapidly growing mycobacteria
( Mycobacterium fortuitum group, Mycobacterium chelonae , and Mycobacterium abscessus) , Mycobacterium avium complex, Mycobacterium kansasii , and Mycobacterium marinum , as well as Nocardia species and other aerobic
Actinomycetes .
CLSI in vitro susceptibility testing and guidelines for microbroth dilution consists of a panel of antimicrobial agents, including macrolides, aminoglycosides, fluoroquinolones, cefoxitin, imipenem, linezolid, tigecycline, doxycycline, minocycline and trimethoprim-sulfamethoxazole. The CLSI M24-A broth microdilution technique is performed in broth
Müeller-Hinton broth, with a final concentration of microbial inoculum of 1x10 4 to 5x10 4 cfu/ml, incubation at 30ºC and reading after 72 hours. Quality controls are those recommended by the CLSI M24-A document with the addition of other inhouse controls. Mycobacterium peregrinum ATCC 700686 and Enterococcus faecalis ATCC 29212, are to be tested on the RGM plate. The interpretation criteria to class a strains as being susceptible or resistant are included in the mentioned document, except for tigecycline which must be interpreted following the recommendations of the European
Committee on Antimicrobial Susceptibility Testing (EUCAST) [150]. Other useful methods are Sensititre broth microdilution, E-test, Kirby Bauer disk diffusion, flow cytometry and radiometric ones [151-154]. The use of methods such as agar diffusion with disks is not recommended, because they are not well standardized and the results do not correlate well with those of the reference method.
Susceptibility profiles of clinically significant RGM species may be useful in differentiating the M. fortuitum group from the M. chelonae group. Members of the M. fortuitum group are usually susceptible to ciprofloxacin and ofloxacin, while M. chelonae and M. abscessus are resistant to these agents. The same applies to cefoxitin and tobramycin, M. chelonae , characterizes by high MICs of cefoxitin (>64 mg/L) and susceptibility to tobramycin (MIC ≤ 4 mg/L), whereas M. abscessus shows lower MICs of cefoxitin ( ≤ 64 mg/L) and resistance to tobramycin (MIC of >8 mg/L).
6. Treatment of diseases caused by rapidly growing mycobacteria
Identification to the species level is important, because there are predictable antimicrobial susceptibility patterns. Los resultados de nuestro estudio coinciden con los de la mayoría de los trabajos publicados y sugieren que, entre los antimicrobianos disponibles, la amikacina es el más efectivo para el tratamiento de las infecciones producidas por las
MCR, y que M fortuitum es más sensible al conjunto de antimicrobianos que el resto de las especies.Several authors have assessed the susceptibility of some species in different geographic areas, using microdilution in broth and agar methods, but there are few publications in this respect [7,155-158]. Statements of tLa técnica de E-test evaluada por nosotros parece ser útil para determinar la sensibilidad de MCR, sencilla de realizar y asequible a cualquier laboratorio.he Infectious Diseases Society of America and the American Thoracic Society [159] regarding nontuberculous mycobacteria suggest that susceptibility data should be reported and used as a clinical guidance for treatment.
Among the available antimicrobials, aminoglycosides are important parenteral antibiotics used in the treatment of
RGM infections, being amikacin the most active and the most effective drug [36]. Clarithromycin is the second most active drug. Among macrolides, 70% of RGM are susceptible to azithromycin. Clarithromycin has good activity against
M fortuitum but not against M chelonae [50,160]. Mycobacterium abscessus typically shows susceptibility to clarithromycin and azithromycin. Recently, there has been found intrinsic resistance to macrolide antibiotics among several RGM species, such as M mageritense , M boenickei , M goodii , M houstonense , M neworleansense , M porcinum , and Mycobacterium wolinskyi [36,104,161]. Rapidly growing mycobacteria can develop macrolide resistance by specific mutations in the peptidyltransferase region of the 23S ribosome gene [162,163]. Because of this property, we do not recommend the use of monotherapy for infections due to RGM, especially when there is a large organism burden or when macrolide’s MICs are in the 4–8-µg/mL range. Ciprofloxacin and imipenem are also active against most RGM strains except for M. chelonae and M. abscessus [33,36,164,165]. The 8-methoxy fluoroquinolones (gatifloxacin, moxifloxacin) may be effective in some rapidly growing mycobacteria infections [165,166].
The choice of treatment varies according to three main factors: the clinical presentation, the implicated mycobacterial species and the immune status of the patient. In vitro resistance to most first-line TB drugs justified, until recently, the need for drug treatment associations [167]. There are no clinical trials comparing different treatment regimens, but in order to select an appropriate treatment, due to the variability in susceptibility among species, it seems necessary to perform an in vitro testing in all significant samples, including the vast majority of traditional antibiotics. Skin infection usually resolves spontaneously or sometimes after surgical debridement. For major infections it is recommended the use of intravenous amikacin (10-15 mg/kg in 2 doses) and cefoxitin (1-2 g iv every 6-8 h for continuous infusion) for a minimum of two weeks. Imipenem is a reasonable alternative to cefoxitin if the mycobacterium is resistant to this drug.
In severe infections treatment must prolong a minimum of 4 months or 6 months in the case of bone infections. For lung infections 6-12 months of treatment could be enough.
Table 3 lists the recommended antimicrobial agents for the treatment of RGM infections and the treatment regimens normally used [7].
370 ©FORMATEX 2011
Science against microbial pathogens: communicating current research and technological advances
_______________________________________________________________________________
Table 3 . Antimicrobial agents used to treat infections due to rapidly growing mycobacteria (Modified from reference [21]).
Antimicrobial agents
Cefoxitin
Imipenem
Amikacin
Tobramycin
Clarithromycin
Azithromycin
Ciprofloxacin
Treatment regimen
1-2 g iv every 6-8 h (for continuous infusion) or 200 mg/kg maximum
12 g/day
0.5 g iv every 6 h or 1 mg iv every
12 h
10-15 mg/kg iv 3 times per week to achieve levels of 20-40 mg/L
2.5 mg/kg iv every 12 h
500 mg orally twice daily or 15-30 mg/kg maximum 1g/day
250-500 mg orally daily or 3 times per week
500-750 mg orally twice daily
Possible activity
Mycobacterium abscessus
Mycobacterium abscessus
Mycobacterium chelonae
Mycobacterium abscessus
Mycobacterium chelonae
Mycobacterium abscessus
Mycobacterium chelonae
Mycobacterium fortuitum
Mycobacterium abscessus
Mycobacterium chelonae
Mycobacterium fortuitum
Mycobacterium abscessus
Mycobacterium chelonae
Mycobacterium fortuitum
Doxycycline/Minocyclyne
Trimethroprim-sulfamethoxazole
100 mg orally twice daily
1 double-strength tablet twice daily
Mycobacterium abscessus
Mycobacterium chelonae
Mycobacterium fortuitum
Mycobacterium smegmatis
Linezolid
Tigecycline
600 mg iv or orally daily
50 mg iv twice daily
Mycobacterium fortuitum
Mycobacterium chelonae
Mycobacterium abscessus
The treatment of infections by M. marinum might involved the surgical excision of the lesion in some cases or the use of different antibiotic regimens with classical tuberculostatics as rifampicin or ethambutol, or other antibiotics such as minocycline, tetracycline, cotrimoxazole, or more recently, levofloxacin or clarithromycin. Spontaneous recovery has been rarely described [168].
En publicaciones recientes se postula la utilización de nuevos antimicrobianos para el tratamiento de las infecciones por micobacterias atípicas, entre los que se citan las nuevas quinolonas, linezolid, tigeciclina, telitromicina o isepamicina.Recent publications postulates the use of new drugs for the treatment of atypical mycobacterial infections, including the new quinolones, linezolid, tigecycline, telithromycin or isepamicin. La actividad in vitro de estos fármacos parece ser buena pero la experiencia clínica aún es limitada.The in vitro activity of these drugs appears to be good but clinical experience is still limited [154,156,166,169-171]. Según nuestros resultados, tigeciclina presenta una excelente actividad para todas las especies de MCR; linezolid es efectivo frente a M chelonae, pero algo menos frente a
M fortuitum y M abscessus, en el caso de quinupristina/dalfopristina, se puede decir que es ineficaz frente a la mayoría de las especies de MCR 12,18,22-24 .
References
[1] Casal MM, Casal M. Las micobacterias atípicas como patógenos emergentes. Enfermedades Emergentes . 2000;2:220-230.
[2] Esteban J, Fernández Roblas R, Soriano F. Micobacterias de crecimiento rápido en patología humana. Enfermedades Infecciosas y Microbiología Clínica . 2000;18:279-286.
[3] Wallace RJ Jr. Recent changes in taxonomy and disease manifestations of the rapidly growing mycobacteria. European Journal of Clinical Microbiology and Infectious Diseases .1994;13:953-960.
©FORMATEX 2011 371
Science against microbial pathogens: communicating current research and technological advances
______________________________________________________________________________
[4] Tortoli E. Impact of genotypic studies on mycobacterial taxonomy: the new mycobacteria of the 1990s. Clinical Microbiology
Reviews . 2003;16:319-354.
[5] Brown BA, Springer B, Steingrube VA, Wilson RW, Pfyffer GE, Garcia MJ, Menedez MC, Rodrigez-Salgado B, Jost KC, Chiu
SH, Onyi GO, Böttger EC, Wallace RJ Jr .
Mycobacterium wolinskyi sp. nov. and Mycobacterium goodii sp. nov., two new rapidly growing species related to Mycobacterium smegmatis and associated with human wound infections: a cooperative study from the International Working Group on Mycobacterial Taxonomy.
International Journal of Systematic Bacteriology .
1999;49:1493-1511.
[6] Schinsky MF, Morey RE, Steigerwalt AG, Douglas MP, Wilson RW, Floyd MM, Butler ER, Daneshvar MI, Brown-Elliott BA,
Wallace RJ Jr, McNeil MM, Brenner DJ, Brown JM. Taxonomic variation in the Mycobacterium fortuitum third-biovariant complex: Description of Mycobacterium bonickei sp. nov., Mycobacterium houstonense sp. nov., Mycobacterium neworleansense sp. nov., Mycobacterium concordense sp. nov., Mycobacterium brisbanense sp. nov., and recognition of
Mycobacterium porcinum from human clinical isolates . International Journal of Systematic and Evolutionary Microbiology.
2004;54:1653-1667.
[7] De Groote MA, Huitt G. Infections due to rapidly growing mycobacteria. Clinical of Infectious Diseases . 2006;42:1756-1763.
[8] Selvaraju SB, Khan IU Yadav JS. Biocidal activity of formaldehyde and nonformaldehyde biocides toward Mycobacterium immunogenum and Pseudomonas fluorescens in pure and mixed suspensions in synthetic metalworking fluid and saline.
Applied and Environmental Microbiology . 2005;71:542-546.
[9] Phillips MS, von Reyn CF. Nosocomial infections due to nontuberculous mycobacteria. Clinical Infectious Diseases . 2001;
33:1363–1674.
[10] Brown-Elliott BA, Griffith DE, Wallace RJ Jr. Newly described emerging human species of nontuberculosis mycobacteria.
Infectious Disease Clinics of North America . 2002;16:187-220.
[11] Wang SX, Tay L, Sng LH. Rapid identification of pathogenic rapidly growing mycobacteria by PCR-restriction endonuclease analysis. Annals Academy of Medicine Singapore . 2005;34:137-140.
[12] Brown-Elliott BA, Wallace RJ Jr. Clinical and taxonomic status of pathogenic non-pigmented or late-pigmented rapidly growing mycobacteria. Clinical Microbiology Reviews.
2002;15:716-746.
[13] Wagner D, Young LS. Nontuberculous mycobacterial infections: a clinical review. Infection.
2004;32:257-270.
[14] Swanson DS. Central venous catheter–related infections due to nontuberculous mycobacterium species. The Pediatric Infectious
Diasease Journal . 1998;17:163-164.
[15] Reilly AF, McGowan KL. Atypical mycobacterial infections in children with cancer. Pediatric Blood and Cancer . 2004;43:698-
702.
[16] Pien FD, Younoszai BG, Pien BC. Mycobacterial infections in patients with chronic renal disease. Infectious Disease Clinics of
North America . 2001;15:851-876.
[17] García-Agudo R, García-Martos P. Aspectos clinicos y microbiologicos de la peritonitis fungica en diálisis peritoneal.
Nefrología . 2009;29:506-517.
[18] Winthrop KL, Albridge K, South D, Albrecht P, Abrams M, Samuel MC, Leonard W, Wagner J, Vugia DJ. The clinical management and outcome of nail salon–acquired Mycobacterium fortuitum skin infection. Clinical Infectious Diseases .
2004;38:38-44.
[19] Cooksey RC, Jhung MA, Yakrus MA, Butler WR, Adékambi T, Morlock GP, Williams M, Shams AM, Jensen BJ, Morey RE,
Charles N, Toney SR, Jost KC Jr, Dunbar DF, Bennett V, Kuan M, Srinivasan A. Multiphasic approach reveals genetic diversity of environmental and patient isolates of Mycobacterium mucogenicum and Mycobacterium phocaicum associated with an outbreak of bacteremias at a Texas hospital. Applied and Environmental Microbiology . 2008;74:2480-2487.
[20] Uslan DZ, Kowalski TJ, Wengenack NL, Virk A, Wilson JW. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Archives of Dermatology . 2006;142:1287-1292.
[21] Chang MA, Jain S, Azar DT. Infections following laser in situ keratomileusis: an integration of the published literature. Survey of Ophthalmology . 2004;49:269–80.
[22] Griffith DE, Girard WM, Wallace RJ Jr. Clinical features of pulmonary disease caused by rapidly growing mycobacteria:
Analysis of 154 patients. The American Review of Respiratory Disease . 1993;147:1271-1278.
[23] Wilson RW, Steingrube VA, Böttger EC, Springer B, Brown-Elliott BA, Vincent V, Jost KC Jr, Zhang Y, Garcia MJ, Chiu SH,
Onyi GO, Rossmoore H, Nash DR, Wallace RJ Jr. Mycobacterium immunogenum sp. nov., a novel species related to
Mycobacterium abscessus and associated with clinical disease, pseudo-outbreaks, and contaminated metalworking fluids: an international cooperative study on mycobacterial taxonomy. International Journal of Systematic and Evolutionary
Microbiology . 2001;51:1751-1764.
[24] Wallace RJ Jr, Zhang Y, Wilson RW, Mann L, Rossmoore H. Presence of a single genotype of the newly described species
Mycobacterium immunogenum in industrial metalworking fluids associated with hypersensitivity pneumonitis. Applied and
Environmental Microbiology . 2002;68:5580-5584.
[25] Cramer JP, Sudeck H, Burchard GD. Pulmonary infection with rapidly growing mycobacteria in a singer with achalasia: a case report. Journal of Infection.
2007;54:219-221.
[26] Ingram CW, Tanner DC, Durack DT, Kernodle GW Jr, Corey GR. Disseminated infection with rapidly growing mycobacteria.
Clinical Infectious Diseases . 1993;16:463-471.
[27] Clark R, Spector H, Friedmann D, Oldrati K, Young C, Nelson S. Osteomyelitis and synovitis produced by Mycobacterium marinum in a fisherman. Journal of Clinical Microbiology.
1990;28:2570-2572.
[28] Vazquez J, Sobel J. A case of disseminated Mycobacterium marinum infection in an immunocompetent patient. European
Journal of Clinical Microbiology and Infectious Diseases.
1992;11:908-911.
[29] Harth M, Ralph E, Farawi R. Septic arthritis due to Mycobacterium marinum . The Journal of Rheumatology.
1993;21:957-960.
[30] Edelstein H. Mycobacterium marinum skin infections. Archives of Internal Medicine.
1994;154:1359-1364.
[31] Aubry A, Chosidow O, Caumes E, Robert J, Cambau E. Sixty-three cases of Mycobacterium marinum infection: clinical features, treatment, and antibiotic susceptibility of causative isolates. Archives of Internal Medicine . 2002;162:1746-1752.
372 ©FORMATEX 2011
Science against microbial pathogens: communicating current research and technological advances
_______________________________________________________________________________
[32] Parent LJ, Salam MM, Appelbaum PC, Dossett JH. Disseminated Mycobacterium marinum infection and bacteremia in a child with severe combined immunodeficiency. Clinical Infectious Diseases.
1995;21:1325-1327.
[33] Hofling-Lima AL, de Freitas D, Sampaio JL, Leao SC, Contarini P. In vitro activity of fluoroquinolones against Mycobacterium abscessus and Mycobacterium chelonae causing infectious keratitis after LASIK in Brazil. Cornea . 2005;24:730-734.
[34] Sungkanuparph S, Sathapatayavongs B, Pracharktam R. Infections with rapidly growing mycobacteria: report of 20 cases.
International Journal of Infectious Diseases.
2003;7:198-205.
[35] Murillo J, Torres J, Bofill L, Ríos-Fabra A, Irausquin E, Istúriz R, Guzmán M, Castro J, Rubino L, Cordido M. Skin and wound infection by rapidly growing mycobacteria: an unexpected complication of liposuction and liposculpture. The Venezuelan
Collaborative Infectious and Tropical Diseases Study Group. Archives of Dermatology . 2000;136:1347-1352.
[36] Han XY, Dé I, Jacobson KL. Rapidly growing gycobacteria. Clinical and microbiologic studies of 115 cases. American Journal of Clinical Pathology . 2007;128:612-621.
[37] Al-Benwan K, Ahmad S, Mokaddas E, Johny M, Kapoor MM. Diagnosis of endocarditis caused by Mycobacterium abscessus .
Annals of Saudi Medicine . 2010;30:408-411.
[38] Sugimoto H, Ito M, Hatano M, Nakanishi Y, Maruyama Y, Yoshizaki T. A case of chronic otitis media caused by
Mycobacterium abscessus . Auris, nasus, larynx . 2010;37:636-639.
[39] Yamamoto A, Hattori T, Shimada H, Mori R, Kazato Y, Yuzawa M. Mycobacterium abscessus corneal ulcer following sutured clear corneal cataract incision. Japanese Journal Of Ophthalmology . 2010;54:499-500.
[40] Renaud CJ, Subramanian S, Tambyah PA, Lee EJ. The clinical course of rapidly growing nontuberculous mycobacterial peritoneal dialysis infections in Asians: A case series and literature review. Nephrology (Carlton) . 2011;16:174-179.
[41] Wongkitisophon P, Rattanakaemakorn P, Tanrattanakorn S, Vachiramon V. Cutaneous Mycobacterium abscessus infection associated with mesotherapy injection. Case Reports in Dermatology . 2011;3:37-41.
[42] Meyers H, Brown-Elliott BA, Moore D, Curry J, Truong C, Zhang Y, Wallace RJ Jr. An outbreak of Mycobacterium chelonae infection following liposuction. Clinical Infectious Diseases.
2002;34:1500-1507.
[43] Wallace RJ Jr, Brown BA, Onyi GO. Skin, soft tissue, and bone infection due to Mycobacterium chelonae chelonae . Importance of prior resistance to oral antimicrobials other than clarithromycin. Journal of Infectious Diseases . 1992;166:405-412.
[44] Garg P, Athmanathan S, Rao GN. Mycobacterium chelonae masquerading as Corynebacterium in a case of infectious keratitis:
A diagnostic dilemma. Cornea .
1998;7:230-232.
[45] Lalitha P, Rathinam SR, Srinivasan M. Ocular infections due to non-tuberculous Mycobacteria. Indian Journal of Medical
Microbiology.
2004;22:231-237.
[46] Regnier S, Cambau E, Meningaud JP, Guihot A, Deforges L, Carbonne A, Bricaire F, Caumes E. Clinical management of rapidly growing mycobacterial cutaneous infections in patients after mesotherapy. Clinical Infectious Diseases . 2009;49:1358-
1364.
[47] Palmero DJ, Ambroggi MG, Poggi SE. Mastitis por Mycobacterium fortuitum en una paciente HIV Negativa. Medicina (Buenos
Aires) . 2004;64:529-532.
[48] Hemmersbach-Miller M, Cárdenes-Santana MA, Conde-Martel A, Bolaños-Guerra JA, Campos-Herrero MI. Cardiac device infections due to Mycobacterium fortuitum . The Canadian Journal of Infectious Diseases and Medical Microbiology .
2005;16:183-185.
[49] Zainal Muttakin AR, Tan AM. Mycobacterium fortuitum catheter-related sepsis in acute leukaemia. Singapore Medical Journal .
2006;47:543-545.
[50] Al-Soub H, Al Maslamani M, Al Khuwaiter J, El Deeb Y, Abu Khattab M. Myocardial abscess and bacteremia complicating
Mycobacterium fortuitum pacemaker infection: case report and review of the literature. The Pediatric Infectious Diasease
Journal. 2009;28:1032-1034.
[51] Hod T, Kushnir R, Paitan Y, Korzets Z. Mycobacterium fortuitum infection in continuous ambulatory peritoneal dialysis.
Clinical Nephrology . 2008;70:546-553.
[52] Guevara-Patiño A, Sandoval de Mora M, Farreras A, Rivera-Olivero I, Fermin D, de Waard JH. Soft tissue infection due to
Mycobacterium fortuitum following acupuncture: a case report and review of the literature. Journal of Infection in Developing
Countries . 2010;4:521-525.
[53] Quiñones C, Ramalle-Gómara E, Perucha M, Lezaun ME, Fernández-Vilariño E, García-Morrás P, Simal G. An outbreak of
Mycobacterium fortuitum cutaneous infection associated with mesotherapy. Journal of the European Academy of Dermatology and Venereology . 2010;24:604-606.
[54] Adékambi T, Foucault C, La Scola B, Drancourt M. Report of two fatal cases of Mycobacterium mucogenicum central nervous immunocompetent patients. Journal of Clinical Microbiology . 2006;44:873-840.
[55] Fleming G, Frangoul H, Dermody T, Halasa N. A cord blood transplant recipient with Mycobacterium mucogenicum central venous catheter infection after infucion of tap water. The Pediatric Infectious Diasease Journal . 2006;25:567-569.
[56] Adékambi T. Mycobacterium mucogenicum group infections: a review. Clinical Microbiology and Infection . 2009;15:911-918.
[57] Shachor-Meyouhas Y, Sprecher H, Eluk O, Ben-Barak A, Kassis I. An outbreak of Mycobacterium mucogenicum bacteremia in pediatric hematology-oncology patients. The Pediatric Infectious Diasease Journal . 2010;30:30-32.
[58] Esteban J, Fernández-Roblas R, Román A, Molleja A, Jiménez MS, Soriano F. Catheter-related bacteremia due to
Mycobacterium aurum in an immunocompromised host. Clinical Infectious Diseases.
1998; 26:496-497.
[59] Koranyi KI, Ranalli MA. Mycobacterium aurum bacteremia in an immunocompromised child, The Pediatric Infectious
Diasease Journal . 2003;22:1108-1109.
[60] Martín-Aspas A, Guerrero-Sánchez F, García-Martos P, González-Moya E, Medina-Varo F, Girón González JA. Bilateral pneumonia by Mycobacterium aurum in a patient receiving infliximab therapy. Journal of Infection . 2008;57:167-169.
[61] Cooksey RC, de Waard JH, Yakrus MA, Rivera I, Chopite M, Toney SR, Morlock GP, Butler WR. Mycobacterium cosmeticum sp. nov., a novel rapidly growing species isolated from a cosmetic infection and from a nail salon. International Journal of
Systematic and Evolutionary Microbiology . 2004;54:2385-2391.
©FORMATEX 2011 373
Science against microbial pathogens: communicating current research and technological advances
______________________________________________________________________________
[62] Moreno Guillen S, Sanz Hospital J, Gomez Mampaso E, Guerrero Espejo A, Ezpeleta Baquedano C, Ortega Calderon A.
Gluteal abscess caused by Mycobacterium flavescens . Tubercle. 1986;67:151-153.
[63] Allen DM, Chang HH. Disseminated Mycobacterium flavescens in a probable case of chronic granulomatous disease. Journal of
Infection . 1993;26:83-86.
[64] Mastroianni A. Mycobacterium flavescens vertebral osteomyelitis in an immunocompetent host. Le Infezioni in Medicina .
2003;11:97-101.
[65] Davison MB, McCormack JG, Blacklock ZM, Dawson DJ, Tilse MH, Crimmins FB. Bacteremia caused by Mycobacterium neoaurum . Journal of Clinical Microbiology . 1988;26:762-764.
[66] Woo PC, Tsoi HW, Leung KW, Lum PN, Leung AS, Ma CH, Kam KM, Yuen KY. Identification of Mycobacterium neoaurum isolated from a neutropenic patient with catheter-related bacteremia by 16S rRNA sequencing. Journal of Clinical
Microbiology . 2000; 38:3515-3517.
[67] Heckman GA, Hawkins C, Morris A, Burrows LL, Bergeron C.Rapidly progressive dementia due to Mycobacterium neoaurum meningoencephalitis. Emerging Infectious Diseases. 2004;10:924-927.
[68] Van Duin D, Goldfarb J, Schmitt SK, Tomford JW, Tuohy MJ, Hall GS. Nontuberculous mycobacterial blood stream and cardiac infections in patients without HIV infection. Diagnostic Microbiology and Infectious Disease . 2010;67:286-290.
[69] Rubia MF, Chozas N, García-Martos P, Reyes F. Bacteriemia por Mycobacterium neoaurum en un paciente inmunodeprimido.
Enfermedades Infecciosas y Microbiología Clínica . 2009;27:58-59.
[70] Brown-Elliott BA, Wallace RJ Jr, Petti CA, Mann LB, McGlasson M, Chihara S, Smith GL, Painter P, Hail D, Wilson R,
Simmon KE. Mycobacterium neoaurum and Mycobacterium bacteremicum sp. nov. as causes of mycobacteremia. Journal of
Clinical Microbiology . 2010;48:4377-4385.
[71] Aguilar JL, Sanchez EE, Carrillo C, Alarcon GS, Silicani A. Septic arthritis due to Mycobacterium phlei presenting as infantile
Reiter’s syndrome. The Journal of Rheumatology . 1989;16:1377-1378.
[72] Spiegl PV, Feiner CM. Mycobacterium phlei infection of the foot: A case report. Foot and Ankle International . 1994;15:680-
683.
[73] Paul E, Devarajan P. Mycobacterium phlei peritonitis: A rare complication of chronic peritoneal dialysis. Pediatric
Nephrology .1998;12:67-68.
[74] Karnam S, Alla VM, Kwon J, Harbert T, Sharma A, Airey K, Mooss A. Mycobacterium phlei , a previously unreported cause of pacemaker infection: Thinking outside the box in cardiac device infections. Cardiology Journal . 2010;17:1-4.
[75] Pennekamp A, Pfyffer GE, Wuest J, George CA, Ruef C. Mycobacterium smegmatis infection in a healthy woman following a facelift: case report and review of the literature. Annals of Plastic Surgery . 1997;39:80-83.
[76] Ergan B, Cöplü L, Alp A, Artvinli M. Mycobacterium smegmatis pneumonia. Respirology . 2004;9:283-285.
[77] Newton JA Jr, Weiss PJ, Bowler WA, Oldfield EC. Soft-tissue infection due to Mycobacterium smegmatis : report of two cases.
Clinical Infectious Diseases.
1993;16:530-533.
[78] Pierre-Audigier C, Jouanguy E, Lamhamedi S, Altare F, Rauzier J, Vincent V, Canioni D, Emile JF, Fischer A, Blanche S,
Gaillard JL, Casanova JL. Fatal disseminated Mycobacterium smegmatis infection in a child with inherited interferon gamma receptor deficiency. Clinical Infectious Diseases.
1997;24:982-984.
[79] Schreiber J, Burkhardt U, Rusch-Gerdes S, Amthor M, Richter E, Zugehor M, Rosahl W, Ernst M.. Non-tubercular mycobacterial infection of the lungs due to Mycobacterium smegmatis . Pneumologie . 2001;55:238-243.
[80] Skiest DJ, Levi ME. Catheter-related bacteriemia due to Mycobacterium smegmatis . South Medical Journal . 1998;91:36-37.
[81] Vonmoos S, Leuenberger P, Beer P, de Haller R. Pleuropulmonary infection caused by Mycobacterium smegmatis.
Case description and literature review. Schweizerische Medizinische Wochenschrift . 1986;116:1852-1856.
[82] Liu F, Andrews D, Wright DN. Mycobacterium thermoresistibile infection in an immunocompromised host. Journal of Clinical
Microbiology . 1984;19:546-547.
[83] Neeley SP, Denning DW. Cutaneous Mycobacterium thermoresistibile infection in a heart transplant recipient. Reviews of
Infectious Diseases . 1989;11:608-611.
[84] Wolfe JM, Moore DF. Isolation of Mycobacterium thermoresistibile following augmentation mammaplasty. Journal of Clinical
Microbiology . 1992;30:1036-1038.
[85] La Bombardi VJ, Shastry L, Tischler H. Mycobacterium thermoresistibile infection following knee-replacement surgery.
Journal of Clinical Microbiology . 2005;43:5393-5394.
[86] Hachem R, Raad I, Rolston KV, Whimbey E, Katz R, Tarrand J, Libshitz H. Cutaneous and pulmonary infections caused by
Mycobacterium vaccae . Clinical Infectious Diseases . 1996;23:173-175. [87] Khatter S, Singh UB, Arora J, Rana T, Seth P.
Mycobacterial infections in human immuno-deficiency virus seropositive patients: role of non-tuberculous mycobacteria. The
Indian Journal of Tuberculosis . 2008;55:28-33.
[88] Viana-Niero C, Lima KV, Lopes ML, Rabello MC, Marsola LR, Brilhante VC, Dirham AM, Leão SC. Molecular characterization of Mycobacterium massiliense and Mycobacterium bolletii in isolates collected from outbreaks of infections after laparoscopic surgeries and cosmetic procedures. Journal of Clinical Microbiology . 2008;46:850-855.
[89] Adékambi T, Drancourt M. Mycobacterium bolletii respiratory infections. Emerging Infectious Diseases . 2009;15:302-305.
[90] Koh WJ, Kwon OJ, Lee NY, Kook YH, Lee HK, Kim BJ. First case of disseminated Mycobacterium bolletii infection in a young adult patient. Journal of Clinical Microbiology . 2009;47:3362-3366.
[91] Al-Waili N, Farber B, Gellman L, Gadaleta D. Isolation of Mycobacterium bolletii from human omentum after laparoscopic gastric banding. Clinical Microbiology and Infection . 2010;16:1561-1563.
[92] Lee SA, Raad II, Adachi JA, Han XY. Catheter-related bloodstream infestion caused by Mycobacterium brumae . Journal of
Clinical Microbiology.
2004;42:5429-5431.
[93] Adékambi T, Stein A, Carvajal J, Raoult D, Drancourt M. Description of Mycobacterium conceptionense sp. nov., a
Mycobacterium fortuitum group organism isolated from a posttraumatic osteitis inflammation. Journal of Clinical
Microbiology . 2006;44:1268-1273.
374 ©FORMATEX 2011
Science against microbial pathogens: communicating current research and technological advances
_______________________________________________________________________________
[94] Liao CH, Lai CC, Huang YT, Chou CH, Hsu HL, Hsueh PR. Subcutaneous abscess caused by Mycobacterium conceptionense in an immunocompetent patient. Journal of Infection . 2009;58:308-309.
[95] Thibeaut S, Levy PY, Pelletier ML, Drancourt M. Mycobacterium conceptionense infection after breast implant surgery, France.
Emerging Infectious Diseases . 2010;16:1180-1181.
[96] Yang HJ, Yim HW, Lee MY, Ko KS, Yoon HJ. Mycobacterium conceptionense infection complicating face rejuvenation with fat grafting. Journal of Medical Microbiology . 2011;60:371-374.
[97] Friedman ND, Sexton DJ. Bursitis due to Mycobacterium goodii, a recently described, rapidly growing mycobacterium. Journal of Clinical Microbiology . 2001;39:404-405.
[98] Ferguson DD, Gershman K, Jensen B, Arduino MJ, Yakrus MA, Cooksey RC, Srinivasan A. Mycobacterium goodii infections associated with surgical implants at Corolado hospital. Emerging Infectious Diseases . 2004;10:1868-1871.
[99] Sohail MR, Smilack JD. Hernia repair mesh-associated Mycobacterium goodii infection. Journal of Clinical Microbiology.
2004;42:2858-2860.
[100] Shelton BG, Flanders WD, Morris GK. Mycobacterium sp. as a possible cause of hypersensitivity pneumonitis in machine workers. Emerging Infectious Diseases. 1999;5:270-273.
[101] Del-Castillo M, Palmero D, Lopez B, Paul R, Ritacco V, Bonvehi P, Clara L, Ambroggi M, Barrera L, Vay C. Mesotherapyassociated outbreak caused by Mycobacterium immunogenum . Emerging Infectious Diseases . 2009;15:357-359.
[102] Sampaio JL, Junior DN, de Freitas D, Höfling-Lima AL, Miyashiro K, Alberto FL, Leão SC. An outbreak of keratitis caused by Mycobacterium immunogenum . Journal of Clinical Microbiology . 2006;44:3201-3207.
[103] Shedd AD 4th, Edhegard KD 2nd, Lugo-Somolinos A. Mycobacterium immunogenum skin infections: two different presentations. International Journal of Dermatology . 2010;49:941-944.
[104] Wallace RJ Jr, Brown-Elliott BA, Hall L, Roberts G, Wilson RW, Mann LB, Crist CJ, Chiu SH, Dunlap R, Garcia MJ,
Bagwell JT, Jost KC Jr. Clinical and laboratory features of Mycobacterium mageritense . Journal of Clinical Microbiology .
2002;40:2930-2935.
[105] Adékambi T, Reynaud-Gaubert M, Greub G, Gevaudan MJ, La Scola B, Raoult D, Drancourt M. Amoebal co-culture of
Mycobacterium massiliense sp. nov. from the sputum of a patient with hemoptoic pneumonia. Journal of Clinical
Microbiology . 2004;42:5493-5501.
[106] Duarte RS, Lourenço MC, Fonseca Lde S, Leão SC, Amorim Ede L, Rocha IL, Coelho FS, Viana-Niero C, Gomes KM, da
Silva MG, Lorena NS, Pitombo MB, Ferreira RM, Garcia MH, de Oliveira GP, Lupi O, Vilaça BR, Serradas LR, Chebabo A,
Marques EA, Teixeira LM, Dalcolmo M, Senna SG, Sampaio JL. Epidemic of postsurgical infections caused by
Mycobacterium massiliense . Journal of Clinical Microbiology . 2009;47:2149-2155.
[107] Nakanaga K, Hoshino Y, Era Y, Matsumoto K, Kanazawa Y, Tomita A, Furuta M, Washizu M, Makino M, Ishii N. Multiple cases of cutaneous Mycobacterium massiliense infection in a "hot spa" in Japan. Journal of Clinical Microbiology .
2011;49:613-617.
[108] Wallace RJ Jr, Swenson JM, Silcox VA, Good RC, Tschen JA, Stone MS. Spectrum of disease due to rapidly growing mycobacteria.
Reviews of Infectiuos Diseases . 1983;5:657-679.
[109] Griffith DE, Wallace RJ Jr. Pulmonary disease due to rapidly growing mycobacteria. Seminars in Respiratory Medicine . 1988;9:505-
513.
[110] Wallace RJ Jr, Musser MJ, Hull SI, Silcox WA, Steele LC, Forrester GD, Labidi A, Selander RK. Diversity and sources of rapidly growing mycobacteria associated with infections following cardiac surgery. Journal of Infectious Diseases .
1989;159:708-716.
[111] Esteban J, Martín-de-Hijas NZ, Fernandez AI, Fernandez-Roblas R, Gadea I, Madrid Study Group of Mycobacteria.
Epidemiology of infections due to nonpigmented rapidly growing mycobacteria diagnosed in an urban area. European Journal of Clinical Microbiology and Infectious Diseases . 2008;27:951-957.
[112] Nagao M, Sonobe M, Bando T, Saito T, Shirano M, Matsushima A, Fujihara N, Takakura S, Iinuma Y, Ichiyama S. Surgical site infection due to Mycobacterium peregrinum : a case report and literature review. International Journal of Infectious
Diseases . 2009;13:209-211.
[113] Idigoras P, Jiménez-Alfaro JA, Mendiola J. Osteomielitis esternal posquirurgica por Mycobacterium porcinum . Enfermedades
Infecciosas y Microbiología Clínica . 2007;25:68-69.
[114] Patil R, Patil T, Schenfeld L, Massoud S. Mycobacterium porcinum peritonitis in a patient on continuous ambulatory peritoneal dialysis. Journal of General Internal Medicine . 2011;26:346-348.
[115] Oh WS, Ko KS, Song JH, Lee MY, Ryu SY,Taek S Kwon KT, Lee JH, Peck KR, Lee NY. Catheter-associated bacteriemia by
Mycobacterium senegalense in Korea. BMC Infectious Diseases . 2005;5:107-111.
[116] Schinsky MF, McNeil MM, Whitney AM, Steigerwalt AG, Lasker BA, Floyd MM, Hogg GG, Brenner DJ, Brown JM.
Mycobacterium septicum sp. nov. a new rapidly growing species associated with catheter-related bacteraemia. International
Journal of Systematic and Evolutionary Microbiology . 2000;50:575-581.
[117] Adékambi T, Drancourt M. Isolation of Mycobacterium septicum from the sputum of a patient suffering from hemoptoic pneumonia. Research in Microbiology . 2006;157:466-470.
[118] Shojaei H, Goodfellow M, Magee JG, Freeman R, Gould FK, Brignall CG. Mycobacterium novocastrense sp. nov. a rapdly growing photochromogenic Mycobacterium . International Journal of Systematic Bacteriology.
1997;47:1205-1207.
[119] N'Guessan K, Vincent V, Nahoua G, Kamate M, Dosso M. Mycobacterium novocastrense isolee des expectorations: contamination ou infection respiratoire non tuberculeuse? Medecine et Maladies Infectieuses . 2006;36:180-181.
[120] Van der Heijden IM, Wilbrink B, Schouls LM, van Embden JD, Breedveld FC, Tak PP. Detection of mycobacteria in joint samples from patients with arthritis using a genus-specific polymerase chain reaction and sequence analysis. Rheumatology .
1999;38:547-553.
[121] Kirschner P, Teske A, Schröder KH, Kroppenstedt M, Böttger EC. Mycobacterium confluentis sp. nov. International Journal of Systematic Bacteriology . 1992;42:257-262.
©FORMATEX 2011 375
Science against microbial pathogens: communicating current research and technological advances
______________________________________________________________________________
[122] Turenne CY, Suchak AA, Wolfe JN, Kabani A, Nicolle LE. Soft tissue infection caused by a novel pigmented, rapidly growing mycobacterium species . Journal of Clinical Microbiology . 2003;41:2779-2782.
[123] Schröder KH, Naumann L, Kroppenstedt RM, Reischl U. Mycobacterium hassiacum sp. nov., a new rapidly growing thermophilic mycobacterium. International Journal of Systematic Bacteriology . 1997;47:86-91.
[124] Richter E, Niemann S, Gloeckner FO, Pfyffer GE, Rusch-Gerdes S. Mycobacterium holsaticum sp. nov. International Journal of Systematic and Evolutionary Microbiology . 2002;52:1991-1996.
[125] Kiska DL, Turenne CY, Dubansky AS, Domachowske JB. First case report of catheter-related bacteremia due to
Mycobacterium lacticola . Journal of Clinical Microbiology . 2004;42:2855-2857.
[126] Hogardt M, Schreff AM, Naumann L, Reischl U, Sing A. Mycobacterium monacense in a patient with a pulmonary tumor.
Japanese Journal of Infectious Diseases . 2008;61:77-78.
[127] Taieb A, Ikeguchi R, Yu VL, Rihs JD, Sharma M, Wolfe J, Wollstein R. Mycobacterium monacense : a mycobacterial pathogen that causes infection of the hand. Journal of Hand Surgery American . 2008;33:94-96.
[128] Curry EM, Yehia M, Roberts S. CAPD peritonitis caused by Mycobacterium rhodesiae . Peritoneal Dialysis International .
2008;28:97-99.
[129] Porres JM. Isolation of Mycobacterium rhodochrous From a Cutaneous Lesion. Archives of Dermatology.
1973;108:411-412.
[130] Schönborn C, Handrick W, Schneider P. Uber den Nachweis von Mycobacterium rhodochrous als Erreger einer Perikarditis bei einem Kleinkind. Zeitschrift für die gesamte innere Medizin und ihre Grenzgebiete . 1975;30:679-684.
[131] Kondo A, Mori K, Iwata J, Tamura M, Yamamoto T, Nakao Y, Maeda M. Caseous necrotic granuloma in the pituitary stalk due to nontuberculous Mycobacteria ( Mycobacterium tokaiense ) infection--case report. Neurologia Medico-Chirurgica
(Tokyo) . 2006;46:80-83.
[132] Ausina V, Luquín M, García-Barceló M, Lanéelle MA, Lévy-Frébault V, Belda F, Prats G. Mycobacterium alvei sp. nov.
International Journal of Systematic Bacteriology. 1992;42:529-535.
[133] Adékambi T, Raoult D, Drancourt M. Mycobacterium barrassiae sp. nov., a Mycobacterium moriokaense group species associated with chronic pneumonia. Journal of Clinical Microbiology . 2006;44:3493-3498.
[134] Jimenez MS, Campos-Herrero MI, Garcia D, Luquin M, Herrera L, Garcia MJ. Mycobacterium canariasense sp. nov.
International Journal of Systematic and Evolutionary Microbiology . 2004;54:1729-1734.
[135] Turenne C, Chedore P, Wolfe J, Jamieson F, May K, Kabani A. Phenotypic and molecular characterization of clinical isolates of Mycobacterium elephantis from human specimens. Journal of Clinical Microbiology . 2002;40:1230-1236.
[136] Tortoli E, Rindi L, Bartoloni A, Garzelli C, Mantella A, Mazzarelli G, Piccoli P, Scarparo C. Mycobacterium elephantis : not an exceptional finding in clinical specimens. European Journal of Clinical Microbiology and Infectious Diseases . 2003;22:427-
430.
[137] Hale YM, Pfyffer GE, Salfinger M. Laboratory diagnosis of mycobacterial infections: new tools and lessons learned. Clinical
Infectious Disease. 2001;33:834-846
[138] Butler WR, Guthertz LS. Mycolic acid analysis by high-performance liquid chromatography for identification of
Mycobacterium species. Clinical Microbiology Reviews. 2001;14:704-726.
[139] Katoch VM, Sharma VD. Advances in the diagnosis of mycobacterial infections. Indian Journal of Medical Microbiology.
1997;15:49-55.
[140] Brunello F, Ligozzi M, Cristelli E, Bonora S, Tortoli E, Fontana R. Identification of 54 mycobacterial species by PCRrestriction fragment length polymorphism analysis of the hsp 65 gene. Journal of Clinical Microbiology . 2001;39:2799-2806.
[141] Da Silva CF, Ueki M, Geiger D, Cardoso S. Hsp 65 PCR-restriction enzyme analysis for identification of Mycobacterium in the clinical laboratory. Revista del Instituto de Medicina Tropical . 2001;43:1-9.
[142] Russo C, Tortoli E, Menichella D. Evaluation of the new GenoType Mycobacterium assay for identification of mycobacterial species. Journal of Clinical Microbiology.
2006;44:333-339.
[143] Steingrube VA, Gibson JL, Brown BA, Zhang Y, Wilson RW, Rajagopalan M, Wallace RJ Jr. PCR amplification and restriction endonuclease analysis of a 65-Kilodalton heat shock protein gene sequence for taxonomic separation of rapidly growing mycobacteria. Journal of Clinical Microbiology . 1995;33:149-153.
[144] Suffys PN, Silva Rocha A, de Oliveira M, Campos CE, Barreto AM, Portaels F, Rigouts L, Wouters G, Jannes G, van
Reybroeck G, Mijs W, Vanderborght B. Rapid identification of mycobacteria to the species level using INNO-LiPA
Mycobacteria, a reverse hybridization assay. Journal of Clinical Microbiology . 2001;39:4447-4482.
[145] Tortoli E, Mariottini A, Mazzarelli G. Evaluation of INNO-LiPA Mycobacteria v2: Improved reverse hybridization multiple
DNA probe assay for mycobacterial identification. Journal of Clinical Microbiology . 2003;41:4418-4420.
[146] Han XY, Pham AS, Tarrand JJ, Sood PK, Luthra R. Rapid and accurate identification of mycobacteria by sequencing hypervariable regions of the 16S ribosomal RNA gene. American Journal of Clinical Pathology. 2002;118:796-801.
[147] Adékambi T, Drancourt M. Dissection of phylogenic relationships among nineteen rapidly growing mycobacterium species by
16S rRNA, hsp65 , sodA , recA , and rpoB gene sequencing. International Journal of Systematic and Evolutionary Microbiology.
2004; 54:2095-2105.
[148] National Committee for Clinical Laboratory Standars. Susceptibility Testing of Mycobacteria, Nocardiae, and other Aerobic
Actinomycetes; Approved Standard NCCLS document M24-A. NCCLS, 940 West Valley Road, Suite 1400, Wayne, PA, USA.
2003.
[149] Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Sixteenth
Informational Supplement. CLSI document M100-S16. CLSI, 940 West Valley Road, Suite 1400, Wayne, PA, USA. 2006.
[150] European Committee on Antimicrobial Susceptibility Testing (EUCAST) Steering Committee. EUCAST technical note on tigecycline. Clinical Microbiology and Infection. 2006;12:1147-1149.
[151] Koontz F. E-test for susceptibility testing of rapid growing mycobacteria. Diagnostic Microbiology and Infectious Disease.
1994; 19:183-186.
[152] Biehle JR, Cavalieri SJ, Saudoble MA, Getsinger LJ. Evaluation of E-test for susceptibility of rapidly growing mycobacteria.
Journal of Clinical Microbiology . 1995;33:1760-1764.
376 ©FORMATEX 2011
Science against microbial pathogens: communicating current research and technological advances
_______________________________________________________________________________
[153] Woods GL, Bergmann JS, Witebsky FG, Fahle GA, Boulet B, Plaunt M, Brown BA, Wallace RJ Jr, Wanger A. Multisite reproducibility of Etest for susceptibility testing of Mycobacterium abscessus , Mycobacterium chelonae , and Mycobacterium fortuitum . Journal of Clinical Microbiology . 2000;38:656-661.
[154] García-Agudo L, García-Martos P, Jesús I, Rodríguez-Iglesias M. Sensibilidad a los antimicrobianos de micobacterias de crecimiento rápido mediante el metodo E-test. Revista Medica de Chile . 2009;137:912-917.
[155] Set R, Rokade S, Agrawal S, Shastri J. Antimicrobial susceptibility testing of rapidly growing mycobacteria by microdilution -
Experience of a tertiary care centre. Indian Journal of Medical Microbiology . 2010;28:48-50.
[156] Fernández-Roblas R, Esteban J, Cabria F, López JC, Jiménez MS, Soriano F. In vitro susceptibilities of rapid growing mycobacteria to telithromycin (HMR 3647) and seven other antimicrobials. Antimicrobial Agents and Chemotherapy .
2000;44:181-182.
[157] Ruiz-Aragón J, García-Agudo L, Flores S, Rodriguez MJ, Marín P, García-Martos P. Sensibilidad a los antimicrobianos de micobacterias de crecimiento rápido. Revista Española de Quimioterapia . 2007;20:429-432.
[158] Gayathri R, Therese KL, Deepa P, Mangai S, Madhavan HN. Antibiotic susceptibility pattern of rapidly growing mycobacteria.
Journal of Postgraduate Medicine . 2010;56:76-78.
[159] American Thoracic Society. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. American Journal of
Respiratory and Critical Care Medicine .
1997;156:S1-25.
[160] Brown-Elliott BA, Wallace RJ Jr, Onyi GO, De Rosas V, Wallace III RJ. Activities of four macrolides, including clarithromycin, against Mycobacterium fortuitum , Mycobacterium chelonae , and Mycobacterium chelonae-like organisms.
Antimicrobial Agents and Chemotherapy.
1992;36:180-184.
[161] Nash KA, Andini N, Zhang Y, Brown-Elliott BA, Wallace RJ Jr. Intrinsic macrolide resistance in rapidly growing mycobacteria. Antimicrobial Agents and Chemotherapy. 2006;50:3476-3478.
[162] Wallace RJ Jr, Meier A, Brown BA, Zhang Y, Sander P, Onyi GO, Böttger EC. Genetic basis for clarithromycin resistance among isolates of Mycobacterium chelonae and Mycobacterium abscessus. Antimicrobial Agents and Chemotherapy .
1996;40:1676-1681.
[163] Vemulapalli RK, Cantey JR, Steed LL, Knapp TL, Thielman NM. Emergence of resistance to clarithromycin during treatment of disseminated cutaneous Mycobacterium chelonae infection: case report and literature review. Journal of Infection .
2001;43:163-168.
[164] Yakrus MA, Hernandez SM, Floyd MM, Sikes D, Butler WR, Metchock B. Comparison of methods for identification of
Mycobacterium abscessus and M chelonae isolates. Journal of Clinical Microbiology. 2001;39:4103-4110.
[165] Brown-Elliott BA, Wallace RJ Jr, Crist CJ, Mann L, Wilson RW. Comparison of in vitro activities of gatifloxacin and ciprofloxacin against four taxa of rapidly growing mycobacteria. Antimicrobial Agents and Chemotherapy. 2002;46:3283-3285.
[166] Rodriguez Diaz JC, Lopez M, Ruiz M, Royo G. In vitro activity of new fluoroquinolones and linezolid against nontuberculous mycobacteria. International Journal of Antimicrobial Agents.
2003;21:585–588.
[167] Yang SC, Hsueh PR, Lai HC, Teng LJ, Huang LM, Chen JM, Wang SK, Shie DC, Ho SW, Luh KT. High prevalence of antimicrobial resistance in rapidly growing mycobacteria in Taiwan. Antimicrobial Agents and Chemotherapy . 2003;47:1958-
1962.
[168] Aubry A, Jarlier V, Escolano S, Truffot-Pernot C, Cambau E. Susceptibility pattern of Mycobacterium marinum.
Antimicrobial
Agents and Chemotherapy. 2000;44:3133-136.
[169] Bosó-Ribelles V, Romá-Sánchez E, Salavert-Lletí M, Hernández-Martí V, Poveda-Andrés JL. La tigeciclina, el primer antibiótico de una nueva clase: las glicilciclinas. Revista Española de Quimioterapia . 2007;20:19-35.
[170] Wallace RJ Jr, Brown-Elliott BA, Crist CJ, Mann LB, Wilson RW. Comparison of the in vitro activity of the glycylcycline tigecycline (formerly GAR-936) with those of tetracycline, minocycline, and doxycycline against isolates of nontuberculous mycobacteria. Antimicrobial Agents and Chemotherapy . 2002;46:3164-3167.
[171] Wallace RJ Jr, Brown-Elliott BA, Ward SC, Crist CJ, Mann LB, Wilson RW. Activities of linezolid against rapidly growing mycobacteria. Antimicrobial Agents and Chemotherapy. 2001;45:764-767.
©FORMATEX 2011 377