ME215-Ch3-R - KFUPM Open Courseware
advertisement

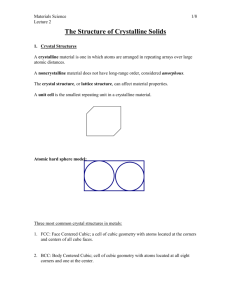
CHAPTER 3 THE STRUCTURE OF CRYSTALLINE SOLIDS Crystal Structures ► Why study the structure of crystalline structure? ► The properties of some materials are directly related to their crystal structure. For example, pure and undeformed magnesium and beryllium, having one crystal structure, are much more brittle than pure and undeformed metals such as gold and silver that have yet another crystal structure. ► Significant property differences exist between crystalline and noncrystalline materials having same composition. Noncrystalline ceramics and polymers normally are optically transparent; the same material in crystalline form tend to be opaque or, at best, translucent. 3.2 Fundamental Concepts ► Chapter 2 was concerned primarily with the various types of atomic bonding, which are determined by the electron structure of the individual atoms. ► Next level of the structure of the materials deals with some of the arrangement that may be assumed by the atoms in the solid state. ► Within this framework, concepts of crystallinity and noncrystallinity are introduced. ► Solid materials may be classified according to the regularity with which atoms or ions are arranged with respect to one another. Crystalline material Î atoms are situated in a repeating or periodic array over large atomic distances ► ► ► ► Upon solidification, the atoms will position themselves in a repetitive three-dimensional pattern, in which each atom is bonded to its nearest-neighbor atoms. All metals, many ceramic materials, and certain polymers form crystalline structure under normal solidification conditions. Noncrystalline or amorphous materials Î do not crystallize. 3.2 FUNDAMENTAL CONCEPTS SOLIDS AMORPHOUS CRYSTALLINE Atoms in a crystalline solid are arranged in a repetitive three dimensional pattern Long Range Order All metals are crystalline solids Atoms in an amorphous solid are arranged randomly- No Order Many ceramics are crystalline solids Some polymers are crystalline solids 3.2 Fundamental Concepts (Contd.) ► When describing crystalline structures, atoms ( or ions ) are thought of as being solid spheres having well-defined diameters. ► This is termed the atomic hard sphere model in which spheres representing nearest-neighbor atoms touch one another. ► An example of the hard sphere model for the atomic arrangement found in some common elemental metals is displayed in Figure 3.1c ► Lattice Î a 3-D array of points coinciding with atom positions or sphere centers. LATTICE Lattice -- points arranged in a pattern that repeats itself in three dimensions. The points in a crystal lattice coincides with atom centers 3-D view of a lattice 3.3 Unit Cells The atomic order in crystalline solids indicates that small groups of atoms form a repetitive pattern. Î in describing crystal structures, it is often convenient to subdivide the structure into small repeat entities called unit cells. ► Unit cell: The basic structural unit or building block of the crystal structure and defines the crystal structure by virtue of its geometry and the atom positions within. ► ► For most crystal structures are parallelepipeds or prisms having three sets of parallel faces ( In case of Figure 3.1c, it is cube ) ► Parallelepiped corners coincides with centers of the hard sphere atoms. ► Generally use the unit cell having the highest level of geometrical symmetry. More than single unit cell may be chosen for a particular crystal structure. Unit cell & Lattice Lattice Unit Cell 3.4 Metallic Crystal Structures ► The atomic bonding in this group of materials is metallic, and thus nondirectional. Î there are no restrictions as to the number and position of nearest-neighbor atoms; this leads to relatively large numbers of nearest neighbors and dense atomic packings for most metallic crystal structures. Have several reasons for dense packing: Typically, only one element is present, so all atomic radii are the same. ► Metallic bonding is not directional. ► Nearest neighbor distances tend to be small in ► order to have lower bonding energy. ► Using the hard sphere model for the crystal structure of metals, each sphere represents an ion core. Table 3.1 presents the atomic radii and crystal structure type. 4 ► Three ► ► ► relatively simple structures found are: Face-Centered Cubic (FCC); Body-Centered Cubic (BCC); Hexagonal Close-Packed (HCP). FACE CENTERED CUBIC STRUCTURE (FCC) 3.4 Metallic Crystal Structures (Contd.) The Face-Centered Cubic Crystal Structure ► Unit cells of cubic geometry, with atoms located at each of the corners and the centers of all the cube faces. ► FCC Î Face-Centered Cubic ► Found in Copper, Aluminum, Silver, and Gold. The spheres or ion cores in FCC touch one another across a face diagonal; the cube edge length a and the the atomic radius R are related through a = 2R√2 ► Each corner atom is shared among eight unit cells, whereas a facecentered atom belong to only two. Î 1/8 of each of the eight corner atoms and ½ of each of the six face atoms, or a total of four whole atoms, may be assigned to a given unit cell. ► FACE CENTERED CUBIC STRUCTURE (FCC) Al, Cu, Ni, Ag, Au, Pb, Pt 3.4 Metallic Crystal Structures (Contd.) The Face-Centered Cubic Crystal Structure ► Two other important characteristics of a crystal structure are: Coordination number and the atomic packing factor (APF). ► Coordination number The number of nearest-neighbor or touching atoms for an atom. For metals, the number is same. For FCC, the coordination number is 12. ► Atomic Packing Factor (APF) APF is the fraction of solid sphere volume in a unit cell, assuming the atomic hard sphere model, or APH = (Volume of atoms in a unit cell ) / (Total unit cell volume ) For FCC, APF=0.74 BODY CENTERED CUBIC STRUCTURE (BCC) 3.4 Metallic Crystal Structures (Contd.) The Body-Centered Cubic Crystal Structure ► BCC Î Body-Centered Cubic ► This metallic crystal structure has a cubic unit cell with atoms located at all eight corners and a single atom at the cube center. ► Center and corner atoms tough one another along cube diagonals. ► Unit cell length (a) and atomic radius (R) are related through a = (4R) / √3 ► Examples: Chromium, iron, tungsten, and others exhibit BCC structure. BODY CENTERED CUBIC STRUCTURE (BCC) Cr, Fe, W, Nb, Ba, V 3.4 Metallic Crystal Structures The Body-Centered Cubic Crystal Structure (Contd.) ► Two atoms are associated with each BCC unit cell. ► The coordination number for BCC is 8. ► Since the coordination number is less for BCC than FCC, so also is the atomic packing factor for BCC lower _____ 0.68 versus 0.74. 3.4 Metallic Crystal Structures The Hexagonal Close-Packed Crystal Structure ► HCP Î Hexagonal Close Packed crystal structure. ► Not all metals have unit cells with cubic symmetry; in HCP it is hexagonal. ► The top and bottom faces of the unit cell consists of six atoms that form regular hexagons and surround a single atom in the center. ► Another plane that provides three additional atoms is situated between the top and the bottom planes. ► The equivalent of six atoms is contained in each unit cell; one-sixth of each of the 12 top and bottom face corner atoms, one-half of each of the 2 center face atoms, and all the 3 midplane interior atoms. 22 September 2003 ME215: Chapter 3 23 HEXAGONAL CLOSE-PACKED STRUCTURE HCP Mg, Zn, Cd, Zr, Ti, Be The Hexagonal Close-Packed Crystal Structure (Contd.) ► If a and c represent, respectively, the short and long unit cell dimensions, c/a ratio should be 1.633. ► The coordination number and APF for HCP crystal structure are same as for FCC: 12 and 0.74, respectively. ► HCP metal includes: cadmium, magnesium, titanium, and zinc. 22 September 2003 ME215: Chapter 3 25 SIMPLE CUBIC STRUCTURE (SC) • Rare due to low packing density (only Pd has this structure) • Close-packed directions are cube edges. Coordination # = 6 (# nearest neighbors) Number of atoms per unit cell BCC 1/8 corner atom x 8 corners + 1 body center atom =2 atoms/uc FCC 1/8 corner atom x 8 corners + ½ face atom x 6 faces =4 atoms/uc HCP 3 inside atoms + ½ basal atoms x 2 bases + 1/ 6 corner atoms x 12 corners =6 atoms/uc Relationship between atomic radius and edge lengths For FCC: For BCC: For HCP a = 2R√2 a = 4R /√3 a = 2R c/a = 1.633 (for ideal case) Note: c/a ratio could be less or more than the ideal value of 1.633 Face Centered Cubic (FCC) 2 a 0 = 4r r 2r r a0 a0 Body Centered Cubic (BCC) 3a 0 = 4r 2a0 a0 3a0 Coordination Number ► The number of touching or nearest neighbor atoms ► SC is 6 ► BCC is 8 ► FCC is 12 ► HCP is 12 ATOMIC PACKING FACTOR APF = Volume of atoms in unit cell* Volume of unit cell *assume hard spheres • APF for a simple cubic structure = 0.52 a R=0.5a close-packed directions contains 8 x 1/8 = 1 atom/unit cell atoms unit cell APF = volume atom 4 π (0.5a)3 1 3 a3 volume unit cell 6 ATOMIC PACKING FACTOR: BCC • APF for a body-centered cubic structure = 0.68 a = 4R /√3 R a Unit cell contains: 1 + 8 x 1/8 = 2 atoms/unit cell atoms volume 4 3 π ( 3a/4) 2 unit cell atom 3 APF = volume a3 unit cell FACE CENTERED CUBIC STRUCTURE (FCC) • Close packed directions are face diagonals. --Note: All atoms are identical; the face-centered atoms are shaded differently only for ease of viewing. • Coordination # = 12 ATOMIC PACKING FACTOR: FCC • APF for a face-centered cubic structure = 0.74 a = 2R√2 a Unit cell contains: 6 x 1/2 + 8 x 1/8 = 4 atoms/unit cell atoms volume 4 3 π ( 2a/4) 4 unit cell atom 3 APF = volume 3 a unit cell 3.5 Density Computations ► ► Density of a material can be determined theoretically from the knowledge of its crystal structure (from its Unit cell information) ► Density= mass/Volume ► Mass is the mass of the unit cell and volume is the unit cell volume. ► mass = ( number of atoms/unit cell) “n” x mass/atom ► mass/atom = atomic weight “A”/Avogadro’s Number “NA” ► Volume = Volume of the unit cell “Vc” THEORETICAL DENSITY # atoms/unit cell ρ = nA Volume/unit cell VcNA (cm3/unit cell) Atomic weight (g/mol) Avogadro's number (6.023 x 1023 atoms/mol) Example problem on Density Computation Problem: Compute the density of Copper Given: Atomic radius of Cu = 0.128 nm (1.28 x 10-8 cm) Atomic Weight of Cu = 63.5 g/mol Crystal structure of Cu is FCC Solution: ρ = n A / Vc NA n= 4 Vc= a3 = (2R√2)3 = 16 R3 √2 NA = 6.023 x 1023 atoms/mol ρ = 4 x 63.5 g/mol / 16 √2(1.28 x 10-8 cm)3 x 6.023 x 1023 atoms/mol Ans = 8.98 g/cm3 Experimentally determined value of density of Cu = 8.94 g/cm3 Densities of Material Classes In general ρmetals > ρceramics > ρpolymers 30 Why? Metals have... Ceramics have... • less dense packing • often lighter elements Polymers have... ρ (g/cm3 ) • close-packing (metallic bonding) • often large atomic masses • low packing density (often amorphous) • lighter elements (C,H,O) Composites have... • intermediate values Metals/ Alloys 20 Platinum Gold, W Tantalum 10 Silver, Mo Cu,Ni Steels Tin, Zinc 5 4 3 2 1 0.5 0.4 0.3 Titanium Aluminum Magnesium Graphite/ Ceramics/ Semicond Polymers Composites/ fibers Based on data in Table B1, Callister *GFRE, CFRE, & AFRE are Glass, Carbon, & Aramid Fiber-Reinforced Epoxy composites (values based on 60% volume fraction of aligned fibers in an epoxy matrix). Zirconia Al oxide Diamond Si nitride Glass -soda Concrete Silicon Ggraphite PTFE Silicone PVC PET PC HDPE, PS PP, LDPE Glass fibers GFRE* Carbon fibers CFRE* Aramid fibers AFRE* Wood Data from Table B1, Callister 7e. 3.6 Polymorphism and Allotropy ► Polymorphism Î The phenomenon in some metals, as well as nonmetals, having more than one crystal structures. ► When found in elemental solids, the condition is often called allotropy. ► Examples: Graphite is the stable polymorph at ambient conditions, whereas diamond is formed at extremely high pressures. Pure iron is BCC crystal structure at room temperature, which changes to FCC iron at 912oC. ► Two or more distinct crystal structures for the same material (allotropy/polymorphism) iron system titanium liquid α, β-Ti 1538ºC δ-Fe BCC carbon 1394ºC diamond, graphite FCC γ-Fe 912ºC BCC α-Fe POLYMORPHISM AND ALLOTROPY BCC (From room temperature to 912 oC) Fe FCC (at Temperature above 912 oC) 912 oC Fe (BCC) Fe (FCC) 3.7 Crystal Systems ► Since there are many different possible crystal structures, it is sometimes convenient to divide them into groups according to unit cell configurations and/or atomic arrangements. ► One such scheme is based on the unit cell geometry, i.e. the shape of the appropriate unit cell parallelepiped without regard to the atomic positions in the cell. ► Within this framework, an x, y, and z coordinate system is established with its origin at one of the unit cell corners; each x, y, and z-axes coincides with one of the three parallelepiped edges that extend from this corner, as illustrated in Figure. The Lattice Parameters Lattice parameters a, b, c, α, β, γ are called the lattice Parameters. ► Seven different possible combinations of edge lengths and angles give seven crystal systems. ► Shown in Table 3.2 ► Cubic system has the greatest degree of symmetry. ► Triclinic system has the least symmetry. 3.7 CRYSTAL SYSTEMS 3.8 Point Coordinates in an Orthogonal Coordinate System Simple Cubic 3.9 Crystallographic Directions in Cubic System Determination of the directional indices in cubic system: Four Step Procedure (Text Book Method) ► Draw a vector representing the direction within the unit cell such that it passes through the origin of the xyz coordinate axes. 2. Determine the projections of the vector on xyz axes. 3. Multiply or divide by common factor to obtain the three smallest integer values. 4. Enclose the three integers in square brackets [ ]. 1. e.g. [uvw] u, v, and w are the integers Crystallographic Directions in Cubic System [111] [120] [110] Crystallographic Directions in Cubic System Head and Tail Procedure for determining Miller Indices for Crystallographic Directions 1. 2. 3. 4. Find the coordinate points of head and tail points. Subtract the coordinate points of the tail from the coordinate points of the head. Remove fractions. Enclose in [ ] Indecies of Crystallographic Directions in Cubic System Direction A Head point – tail point (1, 1, 1/3) – (0,0,2/3) 1, 1, -1/3 Multiply by 3 to get smallest integers 3, 3, -1 A = [33Ī] Direction B Head point – tail point (0, 1, 1/2) – (2/3,1,1) -2/3, 0, -1/2 Multiply by 6 to get smallest integers _ _ B = [403] C = [???] D = [???] Indices of Crystallographic Directions in Cubic System Direction C Head Point – Tail Point (1, 0, 0) – (1, ½, 1) 0, -1/2, -1 Multiply by 2 to get the smallest integers __ C = [0I2] Direction D Head Point – Tail Point (1, 0, 1/2) – (1/2, 1, 0) 1/2, -1, 1/2 Multiply by 2 to get the smallest integers _ D = [I2I] B= [???] A = [???] Crystallographic Directions in Cubic System [210] Crystallographic Directions in Cubic System Crystallographic Directions in Cubic System Indices of a Family or Form < 100 > ≡ [100], [010], [001], [010], [001], [100] < 111 > ≡ [111], [11 1 ], [1 1 1], [ 1 11], [ 1 1 1 ], [ 1 1 1], [ 1 1 1 ], [1 1 1 ] < 110 > ≡ [110], [011], [101], [110], [011], [101] [110], [011], [101], [110], [011], [101] 3.10 MILLER INDICES FOR CRYSTALLOGRAPHIC PLANES ► ► 1. 2. 3. 4. Miller Indices for crystallographic planes are the reciprocals of the fractional intercepts (with fractions cleared) which the plane makes with the crystallographic x,y,z axes of the three nonparallel edges of the cubic unit cell. 4-Step Procedure: Find the intercepts that the plane makes with the three axes x,y,z. If the plane passes through origin change the origin or draw a parallel plane elsewhere (e.g. in adjacent unit cell) Take the reciprocal of the intercepts Remove fractions Enclose in ( ) Miller Indecies of Planes in Crystallogarphic Planes in Cubic System Drawing Plane of known Miller Indices in a cubic unit cell Draw (011) plane Miller Indecies of Planes in Crystallogarphic Planes in Cubic System Origin for A Origin for B Origin for A A = (IĪ0) B = (I22) A = (2IĪ) B = (02Ī) CRYSTALLOGRAPHIC PLANES AND DIRECTIONS IN HEXAGONAL UNIT CELLS Miller-Bravais indices -- same as Miller indices for cubic crystals except that there are 3 basal plane axes and 1 vertical axis. Basal plane -- close packed plane similar to the (1 1 1) FCC plane. contains 3 axes 120o apart. Direction Indices in HCP Unit Cells – [uvtw] where t=-(u+v) Conversion from 3-index system to 4-index system: [u v w ] → [uvtw] ' ' ' n u = (2u ' − v ' ) 3 n ' ' v = ( 2v − u ) 3 t = −(u + v) w = nw Miller Bravais indices are h,k,i,l with i = -(h+k). Basal plane indices (0 0 0 1) ' HCP Crystallographic Directions z Algorithm a2 - a3 1. Vector repositioned (if necessary) to pass through origin. 2. Read off projections in terms of unit cell dimensions a1, a2, a3, or c 3. Adjust to smallest integer values 4. Enclose in square brackets, no commas [uvtw] a2 a1 ex: ½, ½, -1, 0 -a3 a2 2 Adapted from Fig. 3.8(a), Callister 7e. => [ 1120 ] a3 dashed red lines indicate projections onto a1 and a2 axes a1 2 a1 HCP Crystallographic Directions ► Hexagonal Crystals 4 parameter Miller-Bravais lattice coordinates are related to the direction indices (i.e., u'v'w') as follows. z [ u 'v 'w ' ] → [ uvtw ] a2 - a3 a1 Fig. 3.8(a), Callister 7e. 1 u = (2 u ' - v ') 3 1 v = (2 v ' - u ') 3 t = - (u +v ) w = w' Crystallographic Planes (HCP) ► In hexagonal unit cells the same idea is used z example 1. Intercepts 2. Reciprocals a1 1 1 1 1 3. Reduction 4. Miller-Bravais Indices a2 ∞ 1/∞ 0 0 a3 -1 -1 -1 -1 (1011) c 1 1 1 1 a2 a3 a1 Adapted from Fig. 3.8(a), Callister 7e. Miller-Bravais Indices for crystallographic planes in HCP _ (1211) Miller-Bravais Indices for crystallographic directions and planes in HCP Atomic Arrangement on (110) plane in FCC Atomic Arrangement on (110) plane in BCC Atomic arrangement on [110] direction in FCC 3.11 Linear and Planar Atomic Densities ► Linear Density “LD” is defined as the number of atoms per unit length whose centers lie on the direction vector of a given crystallographic direction. Linear Density ► Number of atoms Linear Density of Atoms ≡ LD = Unit length of direction vector [110] a ex: linear density of Al in [110] direction a = 0.405 nm # atoms LD = length 2 2a = 3.5 nm −1 Linear Density LD for [110] in BCC. # of atom centered on the direction vector [110] = 1/2 +1/2 = 1 Length of direction vector [110] = √2 a a = 4R/ √ 3 1 1 3 LD = = = 2a 2 (4 R / 3 ) 2 4R [110] √ 2a Linear Density ► LD of [110] in FCC # of atom centered on the direction vector [110] = 2 atoms Length of direction vector [110] = 4R LD = 2 /4R LD = 1/2R Linear density can be defined as reciprocal of the repeat distance ‘r’ LD = 1/r Planar Density ► Planar Density “PD” is defined as the number of atoms per unit area that are centered on a given crystallographic plane. No of atoms centered on the plane PD = ————————————— Area of the plane Planar Density of (110) plane in FCC # of atoms centered on the plane (110) = 4(1/4) + 2(1/2) = 2 atoms Area of the plane = (4R)(2R √ 2) = 8R22 (111) Plane in FCC PD110 2 atoms 1 = = 2 2 8R 2 4 R 2 a = 2R √ 2 4R Planar Density of (111) Iron Solution (cont): (111) plane 1 atom in plane/ unit surface cell 2a atoms in plane un it atoms above plane rep ea t atoms below plane 2D h= 3 a 2 2 atoms 2D repeat unit Planar Density = area 2D repeat unit ⎛ 4 3 ⎞ 16 3 2 2 area = 2 ah = 3 a = 3 ⎜⎜ R ⎟⎟ = R 3 ⎝ 3 ⎠ 1 16 3 3 atoms = = 7.0 2 R 2 nm 0.70 x 1019 atoms m2 Planar Density of (100) Iron Solution: At T < 912°C iron has the BCC structure. 2D repeat unit (100) Planar Density = area 2D repeat unit 1 a2 = 4 3 R 3 Radius of iron R = 0.1241 nm Adapted from Fig. 3.2(c), Callister 7e. atoms 2D repeat unit a= 1 4 3 3 2 = 12.1 R atoms 19 atoms = 1.2 x 10 nm2 m2 Closed Packed Crystal Structures ► FCC and HCP both have: CN = 12 and APF = 0.74 APF= 0.74 is the most efficient packing. Both FCC and HCP have Closed Packed Planes FCC ----(111) plane is the Closed Packed Plane HCP ----(0001) plane is the Closed Packed Plane The atomic staking sequence in the above two structures is different from each other Closed Packed Structures Closed Packed Plane Stacking in HCP Closed Packed Plane Stacking in FCC Crystalline and Noncrystalline Materials 3.13 Single Crystals ► For a crystalline solid, when the periodic and repeated arrangement of atoms is perfect or extends throughout the entirety of the specimen without interruption, the result is a single crystal. ► All unit cells interlock in the same way and have the same orientation. ► Single crystals exist in nature, but may also be produced artificially. ► They are ordinarily difficult to grow, because the environment must be carefully controlled. ► Example: Electronic microcircuits, which employ single crystals of silicon and other semiconductors. Polycrystalline Materials 3.13 Polycrytalline Materials Polycrystalline Î crystalline solids composed of many small crystals or grains. Various stages in the solidification : a) Small crystallite nuclei Growth of the crystallites. b) Obstruction of some grains that are adjacent to one another is also shown. c) Upon completion of solidification, grains that are adjacent to one another is also shown. d) Grain structure as it would appear under the microscope. Crystals as Building Blocks • Some engineering applications require single crystals: --diamond single crystals for abrasives (Courtesy Martin Deakins, GE Superabrasives, Worthington, OH. Used with permission.) --turbine blades Fig. 8.33(c), Callister 7e. (Fig. 8.33(c) courtesy of Pratt and Whitney). • Properties of crystalline materials often related to crystal structure. --Ex: Quartz fractures more easily along some crystal planes than others. (Courtesy P.M. Anderson) Polycrystals • Most engineering materials are polycrystals. 1 mm • Nb-Hf-W plate with an electron beam weld. • Each "grain" is a single crystal. • If grains are randomly oriented, overall component properties are not directional. • Grain sizes typ. range from 1 nm to 2 cm (i.e., from a few to millions of atomic layers). Anisotropic Adapted from Fig. K, color inset pages of Callister 5e. (Fig. K is courtesy of Paul E. Danielson, Teledyne Wah Chang Albany) Isotropic Single vs Polycrystals • Single Crystals E (diagonal) = 273 GPa Data from Table 3.3, Callister 7e. (Source of data is R.W. Hertzberg, Deformation and Fracture Mechanics of Engineering Materials, 3rd ed., John Wiley and Sons, 1989.) -Properties vary with direction: anisotropic. -Example: the modulus of elasticity (E) in BCC iron: • Polycrystals -Properties may/may not vary with direction. -If grains are randomly oriented: isotropic. (Epoly iron = 210 GPa) -If grains are textured, anisotropic. E (edge) = 125 GPa 200 µm Adapted from Fig. 4.14(b), Callister 7e. (Fig. 4.14(b) is courtesy of L.C. Smith and C. Brady, the National Bureau of Standards, Washington, DC [now the National Institute of Standards and Technology, Gaithersburg, MD].) 3.15 Anisotropy ► The physical properties of single crystals of some substances depend on the crystallographic direction in which the measurements are taken. ► For example, modulus of elasticity, electrical conductivity, and the index of refraction may have different values in the [100] and [111] directions. ► This directionality of properties is termed anisotropy. ► Substances in which measured properties are independent of the direction of measurement are isotropic. SUMMARY • Atoms may assemble into crystalline or amorphous structures. • Common metallic crystal structures are FCC, BCC, and HCP. Coordination number and atomic packing factor are the same for both FCC and HCP crystal structures. • We can predict the density of a material, provided we know the atomic weight, atomic radius, and crystal geometry (e.g., FCC, BCC, HCP). • Crystallographic points, directions and planes are specified in terms of indexing schemes. Crystallographic directions and planes are related to atomic linear densities and planar densities. SUMMARY • Materials can be single crystals or polycrystalline. Material properties generally vary with single crystal orientation (i.e., they are anisotropic), but are generally non-directional (i.e., they are isotropic) in polycrystals with randomly oriented grains. • Some materials can have more than one crystal structure. This is referred to as polymorphism (or allotropy). • X-ray diffraction is used for crystal structure and interplanar spacing determinations.