Point Defects in Metals
advertisement

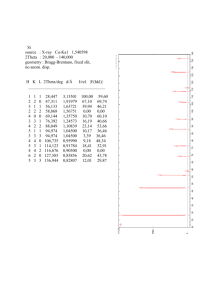
Point Defects in Metals 5.1 Calculate the fraction of atom sites that are vacant for lead at its melting temperature of 327°C (600 K). Assume an energy for vacancy formation of 0.55 eV/atom. Solution In order to compute the fraction of atom sites that are vacant in lead at 600 K, we must employ Equation 5.1. As stated in the problem, Qv = 0.55 eV/atom. Thus, Q Nv 0.55 eV / atom = exp v = exp kT N (8.62 105 eV / atom - K) (600 K) = 2.41 10-5 5.6 Calculate the number of Frenkel defects per cubic meter in zinc oxide at 1000°C. The energy for defect formation is 2.51 eV, whereas the density for ZnO is 5.55 g/cm3 at 1000°C. Solution This problem asks that we compute the number of Frenkel defects per cubic meter in zinc oxide at 1000C. Solution of this problem is possible using Equation 5.3. However, we must first determine the value of N, the number of lattice sites per cubic meter, which is possible using a modified form of Equation 5.2; thus N = N A AZn + AO (6.022 1023 atoms/mol)(5.55 g/cm3)(10 6 cm3/m3) 65.41 g/mol + 16.00 g/mol = 4.11 1028 lattice sites/m3 And, finally the value of Nfr is computed using Equation 5.3 as Q fr N fr = N exp 2kT 2.51 eV = (4.11 1028 lattice sites/m3) exp -5 (2)(8.62 10 eV/K)(1000 + 273 K) = 4.43 1023 defects/m3 Impurities in Solids 5.11 Atomic radius, crystal structure, electronegativity, and the most common valence are tabulated in the following table for several elements; for those that are nonmetals, only atomic radii are indicated. Cu Atomic Radius (nm) 0.1278 C 0.071 H 0.046 O 0.060 Ag 0.1445 FCC 1.9 +1 Al 0.1431 FCC 1.5 +3 Co 0.1253 HCP 1.8 +2 Cr 0.1249 BCC 1.6 +3 Fe 0.1241 BCC 1.8 +2 Ni 0.1246 FCC 1.8 +2 Pd 0.1376 FCC 2.2 +2 Pt 0.1387 FCC 2.2 +2 Zn 0.1332 HCP 1.6 +2 Element Crystal Structure Electronegativity Valence FCC 1.9 +2 Which of these elements would you expect to form the following with copper: (a) A substitutional solid solution having complete solubility (b) A substitutional solid solution of incomplete solubility (c) An interstitial solid solution Solution In this problem we are asked to cite which of the elements listed form with Cu the three possible solid solution types. For complete substitutional solubility the following criteria must be met: 1) the difference in atomic radii between Cu and the other element (R%) must be less than ±15%, 2) the crystal structures must be the same, 3) the electronegativities must be similar, and 4) the valences should be the same, or nearly the same. Below are tabulated, for the various elements, these criteria. Element R% Cu C H O Ag Al Co Cr Fe Ni Pd Pt Zn –44 –64 –53 +13 +12 -2 -2 -3 -3 +8 +9 +4 Crystal Structure Electronegativity FCC FCC FCC HCP BCC BCC FCC FCC FCC HCP Valence 2+ 0 -0.4 -0.1 -0.3 -0.1 -0.1 +0.3 +0.3 -0.3 1+ 3+ 2+ 3+ 2+ 2+ 2+ 2+ 2+ (a) Ni, Pd, and Pt meet all of the criteria and thus form substitutional solid solutions having complete solubility. At elevated temperatures Co and Fe experience allotropic transformations to the FCC crystal structure, and thus display complete solid solubility at these temperatures. (b) Ag, Al, Co, Cr, Fe, and Zn form substitutional solid solutions of incomplete solubility. All these metals have either BCC or HCP crystal structures, and/or the difference between their atomic radii and that for Cu are greater than ±15%, and/or have a valence different than 2+. (c) C, H, and O form interstitial solid solutions. These elements have atomic radii that are significantly smaller than the atomic radius of Cu. 5.43 Determine the ASTM grain size number if 25 grains per square inch are measured at a magnification of 600 . Solution This problem asks that we determine the ASTM grain size number if 8 grains per square inch are measured at a magnification of 600. In order to solve this problem we make use of Equation 5.20: 2 M n 1 N M 2 100 where NM = the number of grains per square inch at magnification M, and n is the ASTM grain size number. Solving the above equation for n, and realizing that NM = 8, while M = 600, we have n log 2(25 * 62 ) 1 =10.81 5.2FE What is the composition, in atom percent, of an alloy that consists of 4.5 wt% Pb and 95.5 wt% Sn? The atomic weights for Pb and Sn are 207.19 g/mol and 118.71 g/mol, respectively. (A) 2.6 at% Pb and 97.4 at% Sn (B) 7.6 at% Pb and 92.4 at% Sn (C) 97.4 at% Pb and 2.6 at% Sn (D) 92.4 at% Pb and 7.6 at% Sn Solution We are asked to compute the composition of an alloy in atom percent. Employment of Equation 5.9 leads to CPb = = CPb ASn 100 CPb ASn CSn APb (4.5)(118.71 g/mol) 100 (4.5)(118.71 g/mol) (95.5)(207.19 g/mol) = 2.6 at% CSn = CSn APb 100 CSn APb CPb ASn = (95.5)(207.19 g/mol) 100 (95.5 )(207.19 g/mol) (4.5)(118.71 g/mol) = 97.4 at% which is answer A (2.6 at% Pb and 97.4 at% Sn).