KEY
advertisement

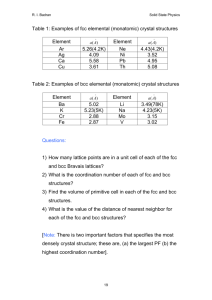
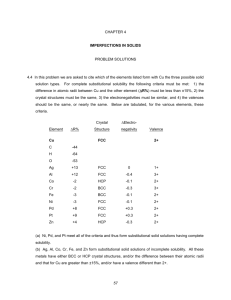
Name: _____KEY________________ CHEM&142_EXAM 1_SPRING 2009_CHAPTERS 20 & 11_100 POINTS Important equations and values on the last page 1. _F__ T/F The greater the IMF of a substance, the greater its vapor pressure. (3 points) 2. _T__T/F The below forms a nonsuperimposable mirror image; i.e., a chiral molecule: (3 points) F I C Br H 3. In each pair circle the best choice (3 points each – 12 points total) a. Lower melting point: propane vs. ethanol b. Greater surface tension: 1,2-ethane-diol vs. water c. Lower viscosity: water vs. methanol d. Greater volatility: methanal vs. methane 4. ___________ typically have a horrid smell similar to wet goat and fetid sweat. (4 points) a. Carboxylic acids b. Cycloalkanes c. Ethers d. Esters 5. Geometric isomers have the same: (4 points) a. Structure b. Number and type of atoms c. None of the above EXAM I Spring09 ALIABADI Page | 1 _________________/26 6. Pick one of the below that correctly describes the packing efficiency of the unit cell from least to greatest. (4 points) a. FCC<BCC<SC b. FCC<SC<BCC c. SC<FCC<BCC d. SC<BCC<FCC 7. According to the phase diagram of sulfur above, there is/are ________ critical temperature/pressure point(s). (4 points) a. One b. Two 8. If monoclinic sulfur is held at a constant pressure of 0.10 mm Hg and the temperature is steadily decreased to 5C, it will (4 points) a. Condense b. Melt c. Sublimate d. None of the above 9. List four unique characteristics of water due to hydrogen bonding: (4 points) HIGH SURFACE TENSION, SPECIFIC HEAT CAPACITY, M.P., AND B.P CAPILLARY ACTION, LIQUID PHASE DENSER THAN SOLID PHASE ____________/16 EXAM I Spring09 ALIABADI Page | 2 10. Why can’t water striders walk on oil? (4 points) NO H-BONDING TO YIELD HIGH SURFACE TENSION 11. Explain why nitrogen is more soluble in water than oxygen. (4 points) NITROGEN ATOMS ARE LARGER THAN OXYGEN ATOMS AND THIS CAUSES A GREATER POLARIZABILITYIN THE FORMER SPECIES 12. Name the following molecules: (5 points each – 10 points total) a. 4-ETHYL-3-METHYLOCTANE O O b. METHYL HEPTANOATE _________________/18 EXAM I Spring09 ALIABADI Page | 3 13. Draw the skeleton structure for the following molecules: (5 points each – 10 points total) a. Diisopropyl ether O b. 3-ethylbenzoic acid O OH 14. Draw the skeletal structures and name the two geometric isomers of 2-hexene: (5 points each – 10 points total) cis-2-hexene trans-2-hexene 15. Crystalline silicon has a cubic structure. The unit cell edge length is 543 pm. The density of the solid is 2.33g/cm3. Calculate the number of silicon atoms in one unit cell. (MW = 28.0856 g/mol) (10 points) 6.022 1023 atoms (543 1010 cm)3 2.33g mol atoms 8.00 3 mol u.cell cm 28.0856 g u.cell ____________/30 EXAM I Spring09 ALIABADI Page | 4 16. Argon crystallized in the FCC arrangement at 40 K. Given that the atomic radius of Ar is 191 pm, calculate the density of solid Ar. (MW = 39.948g/mol) (10 points) FCC: ( 8) r = x = ( 8) 191pm = 540. pm= 540. 10-10 cm 39.948g u.cell mol 4atoms 1.69 g 3 -10 3 23 cm mol (540. 10 cm) 6.022 10 atoms u.cell ________/10 SC: x = 2r BCC: x = 4r/3 FCC: x = (8)r H vap 1 C R T EXAM I Spring09 Ln Pvap - ALIABADI Page | 5