CHEMISTRY 102, General Chemistry Spring 2015
advertisement

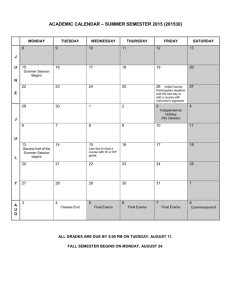
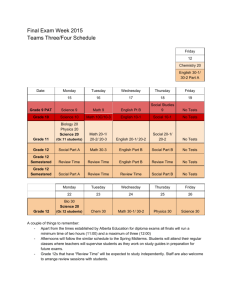
CHEMISTRY 102, General Chemistry Spring 2015 Instructor: Ms. Laura Benton, labenton@email.unc.edu Office: Kenan Labs C142 (next to Chemistry Student Services, near Chemistry Resource Center) Class Times: Tuesday/Thursday, 8:00 – 9:15 AM, Chapman 201 Course Prerequisites: Chem 101, Chem 101L Class Policies Attendance: Not required, but highly recommended if you wish to be successful. You will need to be present to get participation points. Class Websites: Our class website is at http://sakai.unc.edu. Please check regularly for updates. In addition to powerpoints and additional resources for each chapter, there is a forum for you to pose any questions you have on content to your student colleagues. You can also check out my youtube channel, Benton_Chem_101_102, for some videos on how to solve problems. There aren’t too many up yet, but I hope to add more as we go through the semester. Required Textbook: Brown, LeMay & Bursten. Chemistry, the Central Science, 12th edition. The full solutions manual is available in the Chemistry Resource Center. Please read all sections of the textbook that we cover in class. Required Equipment: You will need a cell phone or laptop (with full batteries) to answer Poll Everywhere questions in class. You will also need a good scientific/graphing calculator. Please make sure you have both a backup calculator and extra batteries, especially during exams. You may not use any calculator during exams with wireless, network, or texting capabilities (no cell phones, iPad, iPod, etc). You may not share calculators during exams. Communication: Please come to office hours if you have questions about course content or your performance. If you are unable to attend scheduled hours for some reason, please post content-­‐related questions on the sakai forum. Please do not ask content-­‐related questions over email. Note that by law, I cannot discuss grading issues over email. I am unable to answer emails the day before an exam. Technology: You may use laptop computers to take notes, but please stay on-­‐task. If your cell phone rings in class, I get to answer it. Please keep it on silent. Texting during the lecture distracts your classmates, so please keep all personal cell phone usage to a minimum. Resources for Chem 102 Individual Office Hours: Monday, 2 -­‐ 6 PM, Kenan C142. These hours are for individual meetings to discuss grades and study techniques. Problem-­‐solving questions will be directed to the chemistry resource center. Instructor Recitation Sessions: These optional recitations will be held by me throughout the semester to provide an opportunity for targeted review of background information. A schedule and topics are included in this syllabus. Exam Review Sessions: These optional review sessions will be held prior to the exam. The dates are in the table of recitation sessions included in this syllabus. Mentor Recitations: Undergraduate mentors will be running optional problem-­‐solving recitations throughout the week to go over class topics. You may attend as many of these as you wish. Recitation dates and times will be announced shortly. Prof. Bliem’s Chem 102 Problem-­‐Solving Sessions: These optional sessions are run in the Chemistry Resource Center from 11 AM – 12 PM on Mondays and 12 – 1 PM on Thursdays. If you are struggling with how to solve problems, attending these sessions is a good idea. Chemistry Resource Center: The Chemistry Resource Center (Kenan C143) is open Monday – Thursday from 2 – 7 PM, and has tutors that can help you with problem-­‐solving. Learning Center Science Tutoring: The Learning Center at UNC (learningcenter.unc.edu/) has a plethora of free resources available, including chemistry peer tutoring from 6 – 9 PM on Tuesdays and Wednesdays in Dey Hall, 2nd floor. They also have academic coaches available to meet with students. Make an appointment with one by going to learningcenter.unc.edu. Private Tutors: There is a list of graduate students who are available for hired on the chemistry department website (chem.unc.edu). I also have some folks I can recommend. If you’re starting to struggle, seek help ASAP. If you fail an exam, you are required to meet with me during office hours to discuss your grade. Grading Homework: The problems provided as homework in this syllabus will not be collected or graded. However, I highly recommend that you work through these problems multiple times for your own understanding. A solutions manual is available in the Chemistry Resource Center (Kenan C143). Please do not remove the manual from the resource center. Participation: You will be prompted in-­‐class to answer questions posed in lecture using Poll Everywhere (PE). Your answers to these questions will comprise 7% of your final grade. The total participation points available this semester from PE questions are 250, but the 7% used in grade calculations will be a percentage out of 200 points. This will give you a buffer in case of illness or technical difficulties. Please register for Poll Everywhere according to the directions found at this website: http://help.unc.edu/help/poll-­‐everywhere-­‐faq/ Exams: The remainder of your grade is based on: three hourly exams consisting of short answer questions; and one comprehensive, multiple-­‐choice final. If you have concerns about test-­‐taking, please consult the Learning Center. Please bring your one-­‐card to all exam sessions. Failure to bring your one-­‐card may result in a zero on that exam. The hourly exams will be on February 3rd, March 5th, and April 16th. The final will be on Tuesday, April 28th in our regular classroom at 8 AM. Please bring a scantron to the final exam. Important notes about exams: The hourly exams are optional. The final is required. There are no make –up exams. No exams will be given early. ARS Students: If you are registered with Accessibility Resources and Service, you must schedule your exams to begin at the same time as the rest of the class. Please see me for further instructions on how those exams will be handled. Re-­‐grading: A random selection of exams will be photocopied prior to their return. You may request a re-­‐grading of any exam, but note that any request must be applied to the whole exam, not just one question. Your score after re-­‐grading therefore could be higher OR lower than your original score. If you choose to have your exam re-­‐graded, you must turn in the exam, stapled to the required cover sheet (found on Sakai), no later than one day before the subsequent exam. Exams turned in past this deadline will not be re-­‐graded. Cheating and plagiarism: It is against the UNC honor code to cheat by any means, including looking on someone else’s paper, using cheat sheets/cell phones, passing notes/talking during an exam, etc. Policy adopted by the faculty of the Department of Chemistry on Sept. 9, 1977: "Since all graded work (including homework to be collected quizzes, papers, mid-­‐term examinations, final examinations, research proposals, laboratory results and reports etc.) may be used in the determination of academic progress, no collaboration on this work is permitted unless the instructor explicitly indicates that some specific degree of collaboration is allowed. This statement is not intended to discourage students from studying together or working together on assignments which are not to be collected.” How your final letter grade is determined: • If you take all three hourly exams, the lowest exam grade is dropped, and your final semester grade = 0.07 PE score + 0.25 hour exam + 0.25 hour exam + 0.43 final exam OR = 0.07 PE score + 0.18 hour exam + 0.18 hour exam + 0.57 final exam. I will take whichever one is higher. • If you take two hourly exams, your final grade = 0.07 PE score + 0.18 hourly exam + 0.18 hourly exam + 0.57 final exam. • If you take one hourly exam, your final grade = 0.07 PE score + 0.18 hourly exam + 0.75 final exam. • If you take no hourly exams, your final semester grade = 0.07 PE score + .93 final exam. Those who choose this option rarely earn the grade they want (usually failing). • There will be no changes to the way grades are calculated under this system. You may not, for example, take the hourly exams and then ask for your final exam score to count as your final grade. • The numerical ranges and their corresponding letter grades follows. There are no curves for individual exams. I reserve the right to curve final grades, but only at the end of the course and only if the final class average falls below the C range. Letter Grade Numerical Range A 93 – 100 A-­‐ 90 – 92 B+ 87 – 89 B 83 – 86 B-­‐ 80 – 82 C+ 77 – 79 C 73 – 76 C-­‐ 70 – 72 D 60 – 69 F Below 60 Class Schedule *All dates and topics are subject to change* Date Topic January 8 January 13 January 15 January 20 January 22, 27 January 29 February 3 February 5, 10 February 12, 17, 19 February 24, 26, March 3 March 5 March 10, 12 March 17, 19 March 24, 26 March 31, April 2 April 7 April 9, 14 April 16 April 21, 23 April 28, 8 AM Course Introduction Great Lecture 1: Intermolecular Forces Great Lecture 2: Enthalpy and Entropy Great Lecture 3: Free Energy Chapter 10: Gases Chapter 11: Liquids and Intermolecular Forces Exam 1 Chapter 12: Solids and Modern Materials Chapter 13: Properties of Solutions Chapter 14: Chemical Kinetics Exam 2 SPRING BREAK – no class Chapter 15: Chemical Equilibrium Chapter 16: Acid-­‐Base Equilibria Chapter 17: Additional Aspects of Aqueous Equilibria Chapter 19: Chemical Thermodynamics Chapter 20: Electrochemistry Exam 3 Chapter 21: Nuclear Chemistry Final Exam Optional Recitation Schedule *All dates and topics are subject to change* *Locations and times will be announced in class. * Date Topic Monday, January 12 (Chapman 211, 12:20 PM) Monday, January 26 Thursday, January 29 Thursday, February 5 Monday, February 9 Tuesday, February 10 Tuesday, February 24 Monday, March 2 Tuesday, March 17 Monday, March 23 Tuesday, March 24 Tuesday, March 31 Monday, April 6 Tuesday, April 7 Monday, April 13 Thursday, April 23 Significant Figures Molecular Geometry and Polarity Exam 1 Review Trigonometry Review Molecular Orbital Theory Ionic & Molecular Compounds in Solution Reactions and Kinetics Exam 2 Review Quadratic Equation Review Acid/Base Reactions Logarithms and Sig Figs Titration Review State Functions, Heat, and Work Redox Reactions Exam 3 Review Final Exam Review Homework Problems Reminder: Do not remove the answer key from the resource center for any reason. Again, these problems are assigned and required, but not collected. Please do additional problems in addition to the ones below, should you need the extra practice. Chapter 10: 1, 17, 23, 27, 29, 35, 37, 39, 41, 45, 53, 59, 61, 63, 65, 73, 83, 85, 91, 95, 99 Chapter 11: 11, 13, 17, 19, 21, 23, 27, 35, 37, 39, 43, 45, 51, 59, 61, 63 Chapter 12: 9, 15, 17, 21, 31, 33, 37, 47, 49, 53, 59, 65, 81, 83, 105 Chapter 13: 15, 21, 23, 31, 33, 37, 39, 41, 43, 45, 47, 49, 51, 53, 65, 69, 81, 87 Chapter 14: 9, 19, 21, 23, 25, 27, 29, 31, 33, 35, 37, 43, 47, 51, 57, 61, 65, 73, 75, 85, 91, 93 Chapter 15: 15, 17, 21, 23, 25, 27, 33, 37, 41, 45, 49, 55, 57, 61, 65, Chapter 16: 15, 17, 21, 23, 25, 29, 35, 43, 45, 51, 53, 57, 59, 61, 63, 75, 79, 83, 89, 91, 97 Chapter 17: 17, 19, 21, 23, 27, 29, 37, 41, 43, 45, 51, 53, 59, 61, 67, 71, 73, 77 Chapter 19: 11, 17, 23, 25, 27, 29, 37, 41, 43, 47, 53, 55, 57, 59, 61, 69, 71, 77, 81 Chapter 20: 17, 21, 23, 27, 35, 37, 43, 45, 51, 55, 65, 73, 83, 91, 93 Chapter 21: 7, 9, 11, 13, 15, 17, 19, 21, 27, 29, 35, 39, 41, 43, 47, 49, 51, 53, 59, 61, 67