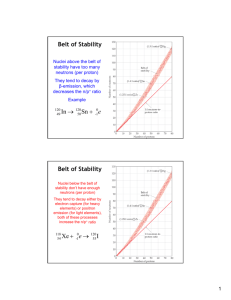
Chapter 21.3 NUCLEAR TRANSMUTATIONS
advertisement

NUCLEAR CHEMISTRY Chapter 21 NUCLEAR CHEMISTRY Introduction The major energy source for our planet • Essential to life on Earth. • Provides ~1 kW/m2. • Used by plants for photosynthesis that produces food for plants and oxygen that most life on Earth needs to survive. The energy of the Sun comes from the hydrogen fusion reaction Nuclear chemistry is the study of nuclear reactions Chapter 21 NUCLEAR CHEMISTRY Nuclear Chemistry Used as therapeutic and diagnostic tools. • Cobalt-60 for cancer therapy (gamma ray) • Fluorine-18 for PET imaging (positron) • Thallium-201 for stress test (gamma ray) Helps determine the mechanisms of chemical reactions Generates electricity Chapter 21 NUCLEAR CHEMISTRY Introduction Figure 21.1 Sources of electricity generation, worldwide and for select countries. Chapter 21 NUCLEAR CHEMISTRY Nuclear Reactions Nuclear power plants (provide 15% of the total electricity in the world) Chapter 21 NUCLEAR CHEMISTRY Nuclear Reactions 국가별 원자력 전력생산량(2009) Chapter 21 NUCLEAR CHEMISTRY The Nucleus Remember that the nucleus is comprised of the two nucleons, protons and neutrons. The number of protons is the atomic number. The number of protons and neutrons together is effectively the mass of the atom. Chapter 21 NUCLEAR CHEMISTRY Isotopes Not all atoms of the same element have the same mass due to different numbers of neutrons in those atoms. There are, for example, three naturally occurring isotopes of uranium: 234 • 92U Uranium-234, trace • 235 92U Uranium-235, 0.7% • 238 92U Uranium-238, 99.3% Chapter 21.1 RADIOACTIVITY Radioactivity A nuclide is a nucleus with a specified number of protons and neutrons It is not uncommon for some nuclides of an element to be unstable, or radioactive. We refer to these as radionuclides. There are several ways radionuclides can decay into a different nuclide. Chapter 21.1 RADIOACTIVITY Types of Radioactive Decay Alpha decay Beta decay Positron emission Gamma decay Electron capture Chapter 21.1 RADIOACTIVITY Alpha Decay -decay is the loss of an -particle (a helium nucleus) 4 2 238 92 U He 234 90 Th + He 4 2 Thorium This expression is called nuclear equation Chapter 21.1 RADIOACTIVITY Beta Decay -decay is the loss of a -particle (a high energy electron). 0 −1 131 53 I or 131 54 0 −1 e Xe + 0 −1 e Chapter 21.1 RADIOACTIVITY Positron Emission Some nuclei decay by emitting a positron, a particle that has the same mass as but an opposite charge to that of an electron. 0 1 11 6 C e 11 5 B + 0 1 e Chapter 21.1 RADIOACTIVITY Electron Capture Addition of an electron to a proton in the nucleus is known as electron capture. • The result of this process is that a proton is transformed into a neutron. p+ e 1 0 1 −1 n 1 0 Chapter 21.1 RADIOACTIVITY Gamma Emission This is the loss of a -ray, which is highenergy radiation that accompanies the loss of a nuclear particle. 0 0 Chapter 21.2 PATTERNS OF NUCLEAR STABILITY Patterns of Nuclear Stability Any element with more than one proton (i.e., anything but hydrogen) will have repulsions between the protons in the nucleus A strong nuclear force helps keep the nucleus from flying apart Neutrons play a key role stabilizing the nucleus Therefore, the ratio of neutrons to protons is an important factor Chapter 21.2 PATTERNS OF NUCLEAR STABILITY Neutron-Proton Ratios For smaller nuclei (Z 20), stable nuclei have a neutron-to-proton ratio close to 1:1. Figure 21.2 Stable and radioactive isotopes as a function of numbers of neutrons and protons in a nucleus. Chapter 21.2 PATTERNS OF NUCLEAR STABILITY Neutron-Proton Ratios As nuclei get larger, it takes a greater number of neutrons to stabilize the nucleus. Figure 21.2 Stable and radioactive isotopes as a function of numbers of neutrons and protons in a nucleus. Chapter 21.2 PATTERNS OF NUCLEAR STABILITY Stable Nuclei The black-dotted region in the figure represent stable, nonradioactive isotopes. Figure 21.2 Stable and radioactive isotopes as a function of numbers of neutrons and protons in a nucleus. Chapter 21.2 PATTERNS OF NUCLEAR STABILITY Stable Nuclei Nuclei above this belt have too many neutrons. They tend to decay by emitting beta particles. Chapter 21.2 PATTERNS OF NUCLEAR STABILITY Stable Nuclei Nuclei below the belt have too many protons. They tend to become more stable by positron emission or electron capture. Chapter 21.2 PATTERNS OF NUCLEAR STABILITY Stable Nuclei There are no stable nuclei with an atomic number greater than 83. Nuclei with such large atomic numbers tend to decay by alpha emission. For example, all isotopes of uranium, atomic number 92, are radioactive. Chapter 21.2 PATTERNS OF NUCLEAR STABILITY Radioactive Series Large radioactive nuclei cannot stabilize by undergoing only one nuclear transformation. They undergo a series of decays until they form a stable nuclide (often a nuclide of lead). Figure 21.3 Nuclear disintegration series for uranium-238. Chapter 21.2 PATTERNS OF NUCLEAR STABILITY Further Observations Nuclei with 2, 8, 20, 28, 50, or 82 protons or 2, 8, 20, 28, 50, 82, or 126 neutrons tend to be more stable than nuclides with a different number of nucleons. (the shell model of the nucleus, magic numbers) Nuclei with an even number of protons and neutrons tend to be more stable than nuclides that have odd numbers of these nucleons. Chapter 21.2 PATTERNS OF NUCLEAR STABILITY Further Observations Figure 21.4 Number of stable isotopes for elements 1–54. Chapter 21.3 NUCLEAR TRANSMUTATIONS Nuclear Transmutations In 1919, Ernest Rutherford performed the first conversion of one nucleus into another. Nuclear transmutations can be induced by accelerating a particle and colliding it with the nuclide. Chapter 21.3 NUCLEAR TRANSMUTATIONS Particle Accelerators These particle accelerators are enormous, having circular tracks with radii that are miles long. Figure 21.5 The Relativistic Heavy Ion Collider. This particle accelerator is located at Brookhaven National Lab on Long Island, New York. Chapter 21.3 NUCLEAR TRANSMUTATIONS Using Neutrons Because Neutrons are neutral, they are not repelled by the nucleus. (acceleration is not required) (radioactive) Transuranium Elements Artificial transformations have been used to produce the elements with atomic number above 92. 2009, Prentice-Hall, Inc. Chapter 21.4 RATES OF RADIOACTIVE DECAY Rates of Radioactive Decay Unaffected by external conditions such as temperature, pressure or state of chemical combination. Figure 21.6 Decay of a 10.0-g sample of Sr-90 (t1/2 = 28.8 yr). Chapter 21.4 RATES OF RADIOACTIVE DECAY Radiometric Dating The half life can serve as a nuclear clock to determine the ages of different objects. A living plant or animal is able to maintain a ratio of 14C to 12C that is nearly identical with that of the atmosphere. Once the organism dies, the ratio of 14C to 12C decreases. The t1/2 of 14C is 5715 yr. Formation: Decay: Chapter 21.4 RATES OF RADIOACTIVE DECAY Radiometric Dating Figure 21.7 Creation and distribution of 14C. The ratio of 14C to 12C in a dead animal or plant is related to the time since death occurred. Chapter 21.4 RATES OF RADIOACTIVE DECAY Kinetics of Radioactive Decay Nuclear transmutation is a first-order process. k is the first order rate constant N is the number of radioactive nuclei The kinetics of such a process, you will recall, obey this equation: (a) (b) (c) One Bq is defined as one nuclear disintegration per second. 1 Ci = 3.7 X 1010 Bq Chapter 21.5 DETECTION OF RADIOACTIVITY Measuring Radioactivity A variety of methods have been devised to detect emissions from radioactive substances Figure 21.8 Badge dosimeters monitor the extent to which the individual has been exposed to high-energy radiation. Chapter 21.5 DETECTION OF RADIOACTIVITY Measuring Radioactivity One can use a device like this Geiger counter to measure the amount of activity present in a radioactive sample. The ionizing radiation creates ions, which conduct a current that is detected by the instrument. Chapter 21.5 DETECTION OF RADIOACTIVITY Measuring Radioactivity Phosphors • Substances excited by radiation can also be used to detect and measure radiation. Scintillation counter • Detects the tiny flashes of light produced when radiation strikes a suitable phosphor. Chapter 21.5 DETECTION OF RADIOACTIVITY Measuring Radioactivity Photographic plates or film. Chapter 21.5 DETECTION OF RADIOACTIVITY Radiotracers How can you prove that plants use CO2 to produce glucose by photosynthesis? What about the source of the oxygen in O 2? Chapter 21.5 DETECTION OF RADIOACTIVITY Radiotracers FDG, fluorodeoxyglucose 18F, t1/2=110min Chapter 21.5 DETECTION OF RADIOACTIVITY Radiotracers Figure 21.10 Schematic representation of a positron emission tomography (PET) scanner. Figure 21.11 Positron emission tomography (PET) scans showing glucose metabolism levels in the brain. Red and yellow colors show higher levels of glucose metabolism. Chapter 21.6 ENERGY CHANGES IN NUCLEAR REACTIONS Nuclear Energy • There is a tremendous amount of energy stored in nuclei. • Einstein’s famous equation, E = mc2, relates directly to the calculation of this energy. • In the types of chemical reactions we have encountered previously, the amount of mass converted to energy has been minimal. • However, these energies are many thousands of times greater in nuclear reactions. Chapter 21.6 ENERGY CHANGES IN NUCLEAR REACTIONS Energy in Nuclear Reactions For example, the mass change for the decay of 1 mol of uranium-238 is −0.0046 g. The change in energy, E, is then E = (m) c2 E = (−4.6 10−6 kg)(3.00 108 m/s)2 E = −4.1 1011 J Chapter 21.6 ENERGY CHANGES IN NUCLEAR REACTIONS Nuclear Binding Energy The mass difference between a nucleus and its constituent nucleons is called the mass defect The energy required to separate a nucleus into its individual nucleons is called the nuclear binding energy Chapter 21.6 ENERGY CHANGES IN NUCLEAR REACTIONS Nuclear Binding Energy The energy required to separate a nucleus into its individual nucleons Figure 21.12 Nuclear binding energies. The average binding energy per nucleon increases initially as the mass number increases and then decreases slowly. Because of these trends, fusion of light nuclei and fission of heavy nuclei are exothermic processes. Chapter 21.7 NUCLEAR POWER: FISSION Nuclear Fission Nuclear fission is the type of reaction carried out in nuclear reactors It is an exothermic process 13 Chapter 21.7 NUCLEAR POWER: FISSION Nuclear Fission 14 Bombardment of the radioactive nuclide with a neutron starts the process. Neutrons released in the transmutation strike other nuclei, causing their decay and the production of more neutrons. Chapter 21.7 NUCLEAR POWER: FISSION Nuclear Fission 14 This process continues in what we call a nuclear chain reaction If there are not enough radioactive nuclides in the path of the ejected neutrons, the chain reaction will die out Chapter 21.7 NUCLEAR POWER: FISSION Nuclear Fission 15 Therefore, there must be a certain minimum amount of fissionable material present for the chain reaction to be sustained: critical mass. Chapter 21.7 NUCLEAR POWER: FISSION Nuclear Fission To trigger a fission reaction, two subcritical masses of uranium-235 are slammed together using chemical explosives 16 Chapter 21.7 NUCLEAR POWER: FISSION Nuclear Fission Chapter 21.7 NUCLEAR POWER: FISSION Nuclear Reactors Nuclear fission produces the energy generated by nuclear power plants Control rods block the paths of some neutrons and regulate the flux of neutrons to prevent the reactor core from overheating 16 Chapter 21.7 NUCLEAR POWER: FISSION Nuclear Reactors In nuclear reactors the heat generated by the reaction is used to produce steam that turns a turbine connected to a generator. Figure 21.19 Basic design of a pressurized water reactor nuclear power plant. Chapter 21.7 NUCLEAR POWER: FISSION Nuclear waste Disposal of spent nuclear fuel poses a major problem in nuclear power The most attractive possibilities appear to be formation of solid materials from the wastes and bury them deep ground in containers of high corrosion resistance and durability Chapter 21.8 NUCLEAR POWER: FUSION Nuclear Fusion The Sun is composed of 73% H, 26%, He, and other elements. Fusion would be a superior method of generating power. • The products of the reaction are not radioactive. • In order to achieve fusion, the material must be in the plasma state at several million kelvins – not practical Requires 40,000,000 K. Chapter 21.9 RADIATION IN THE ENVIRONMENT AND LIVING SYSTEMS Radiations around us Radiation from the Sun Radio waves from radio and TV Microwaves from microwave ovens X-rays from various medical procedures Radiation from the soil and other natural materials Chapter 21.9 RADIATION IN THE ENVIRONMENT AND LIVING SYSTEMS Ionization Radiation Radiation causing ionization (Ionizing radiation) is harmful to biological systems Alpha, beta, and gamma rays/X-rays and higher energy UV radiation can ionize water to form H2O+ Free radical Unstable Highly reactive Chapter 21.8 RADIATION IN THE ENVIRONMENT AND LIVING SYSTEMS Effects of Radiation Figure 21.25 Sources of U.S. average annual exposure to highenergy radiation. The total average annual exposure is 360 mrem. Chapter 21.8 RADIATION IN THE ENVIRONMENT AND LIVING SYSTEMS Radon Radon-222 is a product of the nuclear disintegration series of uranium-238. Radon exposure accounts for more than half of the annual exposure. A noble gas and readily inhaled. Short half life Contributes to 10% of all lung cancer death. Half life = 3.82 days Half life = 3.11 min Chapter 21.8 RADIATION IN THE ENVIRONMENT AND LIVING SYSTEMS Radon Figure 21.26 EPA map of radon zones in the United States. Chapter 21.8 RADIATION IN THE ENVIRONMENT AND LIVING SYSTEMS Radiation Therapy Malignant tumors can be destroyed by exposing them to the same radiation that caused them. Mostly radiation therapy uses the high-energy gamma radiation. M.W. 74.55 M.W. 74.55 21.69 21.77 21.84