Cyclohexanol Oxidation: Chemistry Worksheet
advertisement

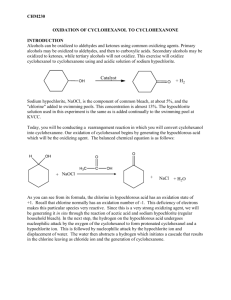
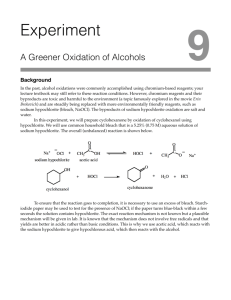
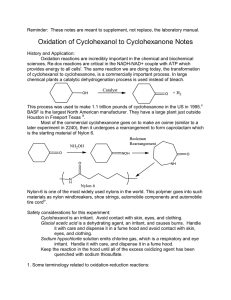
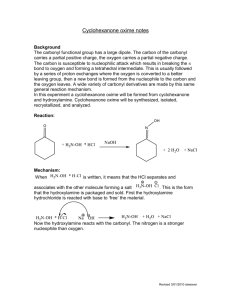
Questions for Experiment 4: Oxidation of Cyclohexanol to Cyclohexanone 1. In the hypochlorite oxidation of cyclohexanol to cyclohexanone, what purpose does the acetic acid serve? 2. Assign peaks at 2939, 2865, and1713 cm-1 in the IR spectrum of cyclohexanone (Figure 22.8, 5th edition). 3. Write a balanced net ionic equation for the reaction of the acidified sodium hypochlorite solution with iodide ion from the starch-iodide paper, assuming that HOCl is reduced to HCl. 4. Describe what your IR spectrum would show if any cylcohexanol remained in the product. 5. Balance the equation for the oxidation-reduction reaction that occurs between bisulfate (HSO3−) and hypochlite (OCl−) to give sulfate and chloride ions.