Los Angeles City College Chemistry 60/68 Mock Exam #3 ANSWER

Chemistry 60/68 Mock Exam #3 ANSWERS pp. 1
5.
4.
Los Angeles City College
Chemistry 60/68 Mock Exam #3 ANSWER KEY
Professor Torres
Multiple Choice
1.
2.
Consider a photon with 4.73 x 10
-19
J of energy. Calculate the wavelength and
frequency.
A.
7.13 x 10
14
nm, 421 Hz
B.
663 nm, 4.52 x 10
14
Hz
C.
663 Hz, 4.52 x 10
14
nm
D.
7.13 x 10
14
Hz, 421 nm
Which set of elements is correctly listed in order of increasing ionization energy?
A. Sb < As < Cl < S < P
B. P < As < Sb < S < Cl
C.
Sb < As < P < S < Cl
D.
Cl < Sb < P < As < S
3. Another way of saying that the energies of electrons are restricted to certain values is to say what?
A. Energies of electrons are quantized.
B. Electrons travel in orbits about the nucleus.
C. Electrons in atoms cannot absorb energy.
D. The location of an electron can be determined.
The region in space within an atom where there is a high probability of finding an electron is called a(an) ____________.
A. main energy level
C. orbital
Which of the following is the correct configuration of electrons in ground state atoms of element number 27?
1 s
2
2 s
2
2 p
6
3s
2
3 p
6
3 d
9
1 s
1
1s
1 s
2
2 s
2
D. 1s
2
2s
2
2
2s
2
2p
2 p
6
3s
2
2p
6
3s
2
6
3s
2
4 s
2
3 p
3p
6
6
4 s
4s
2
2
3 d
4 d
7
7
3d
7
6.
Chemistry 60/68 Mock Exam #3 ANSWERS pp. 2
The common ion of fluorine, F
-
, is isoelectronic with __________.
C. neon
7. Which of the following noble gases will have an atomic radius larger than the atomic radius of argon?
8.
D. both Kr and Xe
If the outermost electron of a neutral potassium atom is removed, the resulting ion will be ___________ the neutral atom.
9.
C. the same size as
D. one half the size of
The hydrogen atom’s spectrum is NOT continuous because electron transitions among the atom’s energy levels _________.
A. involve random amounts of energy
C. involve no energy change
10. A correct ranking of elements in order of decreasing electronegativity is:
A. Al > F > O > Cs > Na
B. Cs > Na > Al > O > F
C. F > O > Al > Na > Cs
D. Na > F > Al > O > Cs
11. According to Hund’s rule, how many unpaired electrons does the ground state of
C. 4
Chemistry 60/68 Mock Exam #3 ANSWERS pp. 3
12. Which of the following forms of electromagnetic radiation has the shortest wavelength and the highest energy?
A.
UV
B.
X-ray
C.
Visible
D.
IR
13. The electron configuration of an atom is 1 s
2
2 s
2
2 p
6
. The atomic number of the
A.
10
B.
11
C.
9
D.
3
14. The term that best describes the electronic geometry of AsH
3
is
A.
linear
B.
planar
C.
tetrahedral
D.
octahedral
15. Which of the following is a polar covalent molecule?
A.
CaCl
2
B.
K
2
O
C.
N
2
D.
CHBr
3
16. Which of the following has the tetrahedral molecular structure?
A.
HBr
B.
CH
4
C.
N
2
D.
CO
2
17. Which compound has double bonds within its molecular structure?
A.
HBr
B.
CH
4
C.
N
2
D.
CO
2
Chemistry 60/68 Mock Exam #3 ANSWERS pp. 4
18. Which of the following is a nonpolar covalent molecule?
A.
CF
4
B.
CO
3
2-
(THIS IS A NONPOLAR COVALENT ION!)
C.
HNO
3
D.
NH
3
For Questions 19 – 22, consider the organic molecule shown below:
B
A H
H C O
C C H
O C C
H C C H
C
O H
19. What is the geometry associated with the carbon labeled “A”?
20. What is the relative bond angle associated with the oxygen labeled “B”?
B. 109.5º
21. What is the relative bond angle associated with the carbon labeled “C”?
C. 120º
22. Select OR “B” for False regarding the following statement: This molecule possesses resonance .
Chemistry 60/68 Mock Exam #3 ANSWERS pp. 5
23. Which of the following four quantum numbers can represent one of the last electrons of cobalt (Co)?
Free Response
A.
n = 3, l = 2, m l
= 0, m s
= ½
B.
n = 4, l = 2, m l
= 0, m s
= ½
C.
n = 3, l = 1, m l
= 0, m s
= ½
D.
n = 3, l = 2, m l
= 0, m s
= 0
E.
n = 3, l = 2, m l
= ½, m s
= ½
24. Draw the best possible Lewis dot structures for each of the listed compounds below, making sure to minimize formal charge(s).
A.
CN
-
C N
B.
NH
4
+
H
H
N H
H
C.
CHCl
3
H
Cl C
Cl
Cl
D.
AlBr
3
Br
Al
Br Br
25. Consider each of the following molecules shown below:
A. Draw the best Lewis dot structures , including resonance where
appropriate.
i
Chemistry 60/68 Mock Exam #3 ANSWERS pp. 6
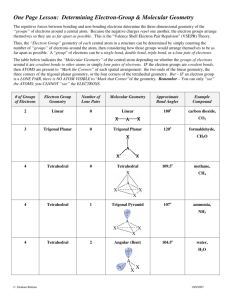
Use theory to draw AND describe the geometry about each
CENTRAL atom. Include both electronic and molecular
geometries.
C. Give the approximate bond angle(s) around each central atom.
D. Determine whether the overall molecule is polar or nonpolar.
Verify your determination using the appropriate VSEPR structure by showing any dipole moments (if any) present.
i.
2
ii.
2
-
NBr
3
iv. Cl
2
S C S
Formal Charge (C) = 0
Electronic Geometry: Linear
Molecular Geometry: Linear
Bond Angle: 180 degrees
Nonpolar ii
H
O
C
O
O
H
C
O
Formal Charge (C) = 0
Electronic Geometry: Trigonal Planar
Molecular Geometry: Trigonal Planar
Bond Angle: approx. 120 degrees
Polar iii.
Br
N
Br
Br
Formal Charge (N) = 0
Electronic Geometry: Tetrahedral
Molecular Geometry: Trigonal Pyramidal
Bond Angle: approx. 109.5 degrees
Polar iv.
Cl C C Cl
Formal Charge (either C) = 0
Electronic Geometry: Linear
Molecular Geometry: Linear
Bond Angle: 180 degrees
Nonpolar
26. Use VSEPR theory to draw the three structural isomers for C
2
H
2
Br
2
. Which isomers are polar/nonpolar? Make sure to clearly label your structures.
***NOTE: Terminal atoms are assumed to have an octet of electrons (excluding hydrogen)—omitted for the sake of clarity!
Chemistry 60/68 Mock Exam #3 ANSWERS pp. 7
H Br H Br H H
C C C C C C
H Br Br H Br
Polar Nonpolar Polar
Br
27. Use Lewis theory AND VSEPR to justify the following observations: NO
3
-1
is nonpolar, while NO
2
-1
is polar.
***NOTE: Terminal atoms are assumed to have an octet of electrons (excluding
O
O
N
O O hydrogen)—omitted for the sake of clarity!
-
O
N
O O
O
N
O
Trigonal planar geometry with resonance around central N gives equivalent N-O bonds where the dipoles DO cross out, resulting in a nonpolar ion.
versus
-
O
N
O O
N
O
Bent geometry with resonance around central N gives equivalent N-O bonds that do NOT cross out, resulting in a polar ion.