Virtual Microscopy in Hematology: experiences from the Belgian EQA
advertisement

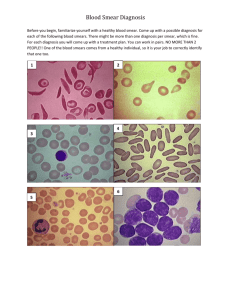
Virtual Microscopy in Hematology: experiences from the Belgian EQA Bernard CHATELAIN U.C.L. Mont-Godinne External Belgian Quality Control in hematomorphology • 3 times a year • Unstained slide fixed in methanol – Laboratory staining procedure • Software on CD-ROM – Wide-field to evaluate red blood cells and platelets morphologies – 210 pictures of nucleated cells to establish a WBC differential count – Clinical information about the case – Calibration procedure and virtual normal smear (105 pictures) Smears: Blood 28 / Bone Marrow 2 2004 November ALL 2005 March Normal, B Prolymphocytic Leukemia 2005 June Normal, Hereditary Spherocytosis 2005 November Normal, Secondary leukemia 2006 March Normal, B12 deficiency 2006 June Normal, Drepanocytosis, May Hegglin (didactic) 2006 November Myeloma, Chediak-Higashi (didactic) B12 deficiency, Secondary leukemia(didactic) (bone marrow aspirate) 2007 March Normal, CML 2007 June Normal, Reactive Lymphocytosis 2007 November Normal, Hairy Cell Leukemia 2008 March Normal, TTP 2008 June Secondary ALL, BiN Polyclonal Hyperlymphocytosis (didactic) 2008 November T Prolymphocytic leukemia, MDS Ider20Q (didactic) 2009 March Hairy Cell Leukemia, t(8;21) AML (didactic) November 2004 March 2005 June 2006 November 2006 May 2008 May 2008 How to reach the”high quality” microscope image How to reach the”high quality” microscope image • Colors RGB colors M R B R + B = M (magenta) White Y C G R + V = Y (yellow) B + V = C (cyan) Retina photoreceptors >>> CCD Rod • Retina: – L cones (Large) => Red (600 à 700 nm) Cone – M (Medium) => Green (500 à 600nm) 2X more frequent – S cones (Short) => Blue (400 à 500nm) • CCD: 1R / 2G / 1B How many levels for a smooth feeling? 200 27 = 128 28 = 256 RGB G How to reach the”high quality” microscope image Gamma = brightness and contrast Gamma dynamic range: eye 24 IL >< CCD 8 IL Gamma curve correction • Increase dynamic range for dark object • But request more than 3 X 8 bit color definition (Olympus DP 71: 11) How to reach the”high quality” microscope image • Image resolution Based on optical resolution you can calculate how many pixels you need! Magnification Numerical Aperture X N.A. L x 2.3 B x 2.3 0.5 0.025 3265 2458 1 0.05 3265 2458 2 0.1 3265 2458 4 0.2 3265 2458 10 0.45 2913 2193 20 0.75 2444 1840 40 0.95 1525 1148 40 1 1623 1222 60 0.95 1017 765 60 1.4 1486 1119 100 1.4 1486 1119 Image pixels density Obj. 100 X NA 1.4 Obj. 40 X NA 1.3 Obj. 100 X NA 1.4 Obj. 40 X NA 1.3 + resampling and accentuation (g=126; r=3.6; s=3.0 How to reach the”high quality” microscope image • Size of the picture Wide field Image Stitching of 80 images (100 x) Obj. 40 X NA 1.3 Material and methods • Olympus PROVIS system – Olympus AX-70 microscope with autofocus and XYZ • Camera – Olympus DP71 – DP manager for image acquisition • • • • Home made software for wide field capture Stitching unlimited (Realviz) to merge pictures Adobe Photoshop for image editing Stitching software for wide-field elaboration www.zoomify.com PID=Pixel On Demand Today maximum size of the digitized field by manual technique Full resolution image (N.A.=1,4 100X equiv) 3 mm / 2 mm Low resolution image (N.A.=0,4 15X equiv) 15 mm / 10 mm Limit is related to the size of the TIF picture (1Gb). And the size of the computer RAM for post stitching correction. Virtual scan www.aperio.com Olympus Dotslide 1 slide= 50 Gb Z-Axis CombineZ picture CombineZ picture Global appreciation of the CD-ROM for the first and second QCs General appreciation of the software and the virtual slide 120 2004-3 2005-1 100 %age of laboratories 80 60 40 20 0 Bad Less good Appreciation against actual smear Good to very good Evaluation of both methods on 5 consecutive assays Cell types Median (total of 5 surveys) Actual smear Virtual smear Diff (Virtual Actual) Polynuclear neutrophils 120 125,2 5,2 Band cells 11 12,65 1,65 Polynuclear neutrophils + Band cells 126 136 10 8 8 0 Lymphocytes 125 130 5 Monocytes 19 20 1 Blast cells 202,5 192,1 -10,4 Other cells 124,7 116 -8,7 Polynuclear eosinophils Relative error (%) 4,15 13,04 7,35 0,00 3,85 5,00 -5,41 -7,50 Actual and virtual smears : diagnosis accuracy for 5 surveys Diagnosis Accuracy : Comparison between actual and virtual smears 100 Actual smear Virtual smear 90 80 Diagnosis accuracy (%) 70 60 50 40 30 20 10 0 2004-3 2005-1 2005-2 Survey 2005-3 2006-1 H/6916 May-Hegglin H/7100 Chediak-Higashi H/7100 MDS ider20q H/9353 AML t(8;21) H/7597 CML Mai 2008 Bone marrow virtual smeal We will try to optimize our material regarding your precious remarks and evaluations Authoring Tool For The External Quality Assessment 1. State of the art BEFORE AFTER Flash Java Content management delegated to a developper Content managed by the experts Dependence on a developper Composition autonomy One domain Any domain Offline Offline - Online No questions - answers mapping Questions – answers mapping (ex. hematology : cell by cell analysis) Answers outside the system Answers inside the system (paper, web forms) (answers analysis possible) 2. Content Logical Representation • Content Transmission Scheme The experts of a domain Authoring Tool ?? Laboratories Logical Content 3. Content Manifestation • Content Transmission Scheme The experts of a domain Authoring Tool OK Laboratories Content Manifestation Logical Content 4. Transformations (Scenario Application) Structured Datas Datas Manifestation (full or partial) Adaptation Transformations Datas Presentation Presentation Transformations XML Applet XSL XSL . . . XML file Browser ... PDF Target Specific FO PDF reader TEXT Text Editor PS Printer . . . ... Smi Presentation Spatial Dimension XSL XSL SMIL Spatio – temporal Dimension Target Independant HTML FOP Processing XML file XSL XSL Adapted Logical Content SMIL Engine General Logical Content Interactive Dimension Program Don’t forget the limitation of classical microscopic slides! Adapted from EQLAM MGG staining recommandations Reagents Sorensen Phosphate Buffer pH 6.8 : Na2HPO4.2H2O M/15 (2.56 g/l) and KH2PO4 M/15 (6.63 g/l) Check the pH of the Sorensen buffer! Buffered water : dilution 1/20e of Sorensen Phosphate Buffer in distilled water May-Grünwald solution Don’t adjust the pH of the buffered water! Giemsa solution Staining Methanol Pure May-Grünwald solution 10 minutes 5 minutes May-Grünwald (50/50 in buffered water buffer pH 6.8) 2-3 minutes Giemsa (1/10 in buffered water pH 6.8) 15 minutes Rinse with running water Buffered water pH 6.8 30 seconds Let dry smears upright Guidelines for blood smear preparation and staining procedure for setting up an external quality assessment scheme for blood smear interpretation. Part I: control material (EQALM), J-L Vives Corrons, S. Albarède, G. Flandrin, S. Heller, K. Hovarth, B. Houwen, G. Nordin, E. Sarkani, M. Skitek, M. Van Blerk, J-C. Libeer, Clin Chem Lab Med, 2004, 42(8):922926 EQALM : External Quality Assurance Programmes in Laboratory Medecine 1 : Darkness + Turbovac 800s (vacuum + N2) + Room temperature 2, 3, 4, 5 : Darkness + Room temperature Stainability alteration during the smear storage Oxygen depletion + N2 injection Turbovac (800s) 1. Packaging of the opaque box containing smears in a plastic bag 2. 98-99 % vacuum 3. Nitrogen gas injection 4. Heat closure of the plastic bag 5. Storage at room temperature MGG staining Immediate staining Staining after 1 ½ month H/2654 (- Turbovac) H/6517 (+ Turbovac) e-Hematimage.eu 50 belgian participants/more than 2500 around the world many thanks for their contribution to Marc Chatelain Yvan Cornet Agathe Debliquis Damien Denizza Hubert Meurisse Jan Philippé Jérôme Pruvot Marjan Van Blerk and the hematology expert comitee Jean-Claude Libeer