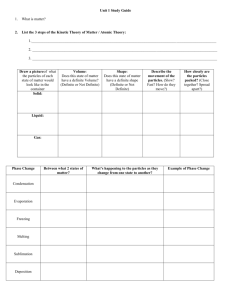
L Matter is anything that has mass and takes up space. Mass is the
advertisement

L Matter is anything that has mass and takes up space. Mass is the amount of mass an object contains. Mass is measured using a scale. Volume is the amount of space matter takes up. Volume can be measured by multiplying the length, width, and height of an object, or by using a measuring cup and seeing how the measurement of water changes in a cup. Density is the amount of matter an object has in a certain space. To find density, divide the mass of an object by the volume of an object. D=M÷V. A property is something that describes matter. Physical properties are things that you can tell about an object using your senses and without changing the object. Example: a ball is round. Chemical properties are things you can tell about an object by changing it into something new that cannot be changed back. Example: wood can burn. The three states of matter are solid, liquid, and gas. A solid has definite shape and definite volume. A liquid has definite volume, but no definite shape. A gas has no definite volume and no definite shape. An element is any material made up of a single kind of matter. Examples of elements are gold, iron, and oxygen. An atom is the smallest part of an element that still has that element’s properties. A chemical symbol is one or two letter that stand for the name of an element. Examples: O stands for oxygen, Fe stands for Iron, H stands for hydrogen, and Au stands for gold. A compound is a kind of matter made up of two or more elements that are joined together and cannot be separated. Examples: water is made of hydrogen and oxygen, and salt is made of sodium and chloride. A molecule is the smallest part of a compound that still has that compounds properties. A chemical formula is a group of symbols that shows the kinds and number of atoms in a single molecule of a compound. Examples: H2O is water and CO2 is carbon dioxide. A physical change is a change in the size, shape, or state of matter in which nothing new is made. Example: tearing a sheet of paper. A mixture is matter made of different substances that can easily be separated. Example: a salad. Energy is the ability to cause change. A solution is a mixture in which particles of different substances are mixed evenly throughout. Example KoolAid is a solution. A chemical change is a change that results in one or more new kinds of matter forming. Example: iron and oxygen combine to form rust. Standard units are the things we measure with, including meters, liters, and grams. A solvent is the part of the solution that dissolves the other part. Example: the water in Kool-Aid is a solvent. A solute is the part of a solution that dissolves. Example: the powder in Kool-Aid is a solute.