energy transfer in light - Hertfordshire Grid for Learning
advertisement

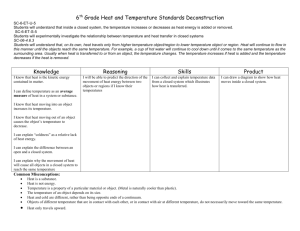
Handout 4.9 Modelling the importance of using energy transfer language when explaining the phenomena of light 1 of 5 This handout provides some possible questions a teacher may ask pupils to develop their understanding of energy through the teaching of light. Two levels of response are provided. The first, in italics, shows what a teacher might say to a pupil in Key Stage 3, while the second, usually longer, provides a more detailed description of the phenomenon as background information for the teacher. 1 Although there is no change to the object, why does the image in a plane mirror dim when the brightness of the illuminating light decreases? When light shines on an object it transfers energy to the object. Some of the light is reflected by the object and the reflected light transfers energy to our eyes. If less energy is transferred to the object by using a dimmer light (say), then there is less available to be transferred to our eyes by the reflected light so the object looks dimmer. When light from a source shines on an object (any object), the light is absorbed by it, reflected from it or transmitted through it. The amount of energy per second arriving at the object must be equal to the total of the amounts per second being absorbed, reflected and transmitted. For any given object and incident light spectrum (i.e. distribution of intensity across wavelengths), the ratio of amounts being absorbed, transmitted and reflected will be the same, whatever the intensity of the incident light. (This may not be true for very large changes in intensity, as the temperature of the object may then change, but it will be true for any moderate change.) So if 20% (say) is reflected when the incident light is relatively dim, then 20% will still be reflected when it is brighter. The brightness of the image of an object depends on the intensity of the light coming from it, i.e. the intensity of the light that is reflected (diffusely) from it. (This is true whether we are talking about the image on our retina or the image on a screen.) The intensity of the light reflected from an object is proportional to the intensity of light incident on it (as explained in the paragraph above). So if you increase the intensity of the illuminating light, the intensity of the reflected light also increases – and therefore so does the brightness of the image. 116 | Strengthening teaching and learning of energy in Key Stage 3 science | Session 4 | Notes for tutors © Crown copyright 2003 Handout 4.9 2 of 5 2 What do you think happens to an object when energy is transferred to it by light? When the energy is transferred to an object by light, one of three things can happen: • it can be transmitted through the object by the transmitted light; • it can be transferred away from the object by the reflected light; • it can be absorbed, raising the temperature of the object. See the first paragraph of the long answer to question 1 above. The energy carried by the fractions of the light that are transmitted and reflected is transferred to whatever eventually absorbs this light. The energy transferred by that fraction of the light that is absorbed in this object usually results in a rise in temperature, that is it ends up stored in the object as internal energy (or thermal energy if you prefer). We say ‘usually’ in the previous sentence because, in a few cases, absorption of light can cause electronic transitions within the material (this is how spectroscopy and microwave ovens work, for example). In this case, the energy is now stored in the molecules of the material the object is made of. It would not be appropriate to introduce this complexity at Key Stage 3 and it would be appropriate to ignore these cases until a basic understanding had been achieved – and simply say, in an introductory treatment, that ‘when an object absorbs radiation, its temperature rises’. Thinking of this in energy terms can be useful – in particular, understanding that absorption of light (and any other electromagnetic radiation) results in an increase in the internal energy of the absorber is valuable – possibly particularly so in the case of absorption of ionising radiation, which many people wrongly think will make the absorber radioactive. 117 | Strengthening teaching and learning of energy in Key Stage 3 science | Session 4 | Notes for tutors © Crown copyright 2003 Handout 4.9 3 of 5 3 When light shines on a glass block, is all the energy transferred through the glass block by the light? (Think – would we be able to see the block if it was?) Some of the light shining on the block is reflected. The reflected light transfers energy to our eyes where it is detected. If all the light were transferred through the block and none was reflected, then there would be no energy transferred to our eyes to be detected and we would not be able to see the block at all. Clearly, some light must be reflected (diffusely) or we would not be able to see the block. The same applies to ‘rays of light’. We only see a ray of light passing through the air or a transparent material if some of the light is diffusely reflected by dust or other impurities in the medium. (Of course we can ‘see’ the ray if we place our eye in a position where the light enters it, i.e. if we look along the ray, towards the source. We are talking above about seeing a ray from the side.) The rule (no exceptions) is: we see objects (all objects) because light comes from them and enters our eye. If the object is luminous, the light originates in or on it. If it is non-luminous, the light has come from another source and is reflected (diffusely) by the object. 4 Do you think the same amount of energy is being transferred into and out of the glass block by light? No. The block absorbs some of the energy and it warms up. No. As explained in the long answer to question 1 above, when light from a source shines on an object (any object), the light is absorbed by it, reflected from it or transmitted through it. Transparent materials allow a large fraction of the incident light to be transmitted through them. But no material is a perfect transmitter of light. Some of the incident light is absorbed by a glass block – and some is reflected from it (as discussed in the answer to question 3 above). The light that is absorbed raises the temperature of the block slightly. In energy terms, some of the energy carried by the incident light has been transferred to the block, where it is now stored as internal (or thermal) energy. 5 Why do coloured filters get hot in use? Coloured filters transmit light of only one colour. The energy transferred to the filter by the other coloured light is absorbed and warms up the filter. This answer uses much the same reasoning as the long answer to question 4 above. No transparent material is a perfect transmitter. Some of the incident light is absorbed by the material. The energy transferred by this light raises the temperature of the material slightly. The only thing that is special about coloured filters is that they are made to absorb some wavelengths of light more than others (and so they also transmit some wavelengths better than others – the ones they haven’t absorbed). 118 | Strengthening teaching and learning of energy in Key Stage 3 science | Session 4 | Notes for tutors © Crown copyright 2003 Handout 4.9 4 of 5 6 Why does cine film melt if the film gets stuck in the projector? The film is like a filter with lots of different coloured patches. Each area absorbs some of the colours of light shining on it and energy is transferred to the film, heating it up. If the film gets stuck, energy is transferred to the same bit of film for a longer time so it heats up more and more until it melts. This can happen very quickly because the projector lamp is very bright and transfers a lot of energy very quickly. The light absorbed by the piece of film that is in the light beam (as explained in the answers to questions 4 and 5 above) raises the film’s temperature. If the film is left for too long in one position, the bit of film being illuminated may get so hot that it melts. If it keeps moving, this doesn’t happen, as the energy transferred to the film is spread over a much longer piece of film, so none of it gets so hot. 7 Why do black objects warm up faster than white ones? Black objects reflect hardly any light at all. All the energy transferred by the light is absorbed by the object, so it warms up quickly. White objects reflect a lot of light so a lot of the energy is transferred away from the object by the reflected light. Less light is absorbed and so less energy is transferred to the object itself, so it warms up more slowly. Black objects absorb all (or in practice almost all) of the light that is incident on them. (In fact, it could be argued that this is a definition of what is meant by ‘black’.) All of the energy carried by the incident light therefore ends up stored in the object, as internal energy (or thermal energy). For non-black objects, only some of the energy carried by the incident light ends up stored in the object as internal energy, so the temperature rise is less. There is a complicating factor here, however, which is that black objects also radiate more energy per second than white ones at the same temperature. If a pupil knows this, he or she may ask why a black object still gets hotter than a white one when light is incident on it. A full explanation needs to consider the net amount of energy gained by the object per second (the difference between the amounts per second being absorbed and transmitted) – this requires several more advanced ideas and a more detailed analysis of the situation, so it might be better avoided at Key Stage 3 (and Key Stage 4). 119 | Strengthening teaching and learning of energy in Key Stage 3 science | Session 4 | Notes for tutors © Crown copyright 2003 Handout 4.9 5 of 5 Addendum: an extra question to think about 8 Is it better to talk of ‘energy carried by a beam of light’ or ‘the energy of a beam of light’? It is sometimes argued that it is not correct to talk about ‘the energy transferred (or carried) by light’, but that light is energy, and so we should talk about ‘the energy of the light’. This is based on the argument that a light beam consists of photons, and that each of these has an amount of energy (E = hf ). This, however, depends on ideas that are well beyond Key Stage 3, and may not be helpful to someone trying to grasp the ideas of light and energy for the first time. There is no object or system in which any measurable amount of energy in the form of light is stored. Instead, we can measure the rate at which energy is being transferred by the light beam (in W/m2). It is better, therefore, to think of light as a mechanism by which energy is transferred from place to place, and not as a way in which energy can be stored. In fact, it can be argued that it is more accurate to say that a photon carries energy E = hf than that it has energy E = hf as the photon has to interact with matter and be absorbed before anything is apparent. Planck and Einstein argued that radiation is emitted and absorbed in quanta. This is an easier idea to grasp first, before wondering about whether or not it therefore travels as quanta – whatever that might mean or imply in observable terms. So, at Key Stage 3 (and Key Stage 4), it would be more helpful to pupils to talk about ‘the energy transferred (or carried) by light’. 120 | Strengthening teaching and learning of energy in Key Stage 3 science | Session 4 | Notes for tutors © Crown copyright 2003