Lab 8: Average Atomic Mass of Halloweenium DATA
advertisement

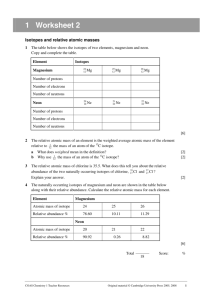
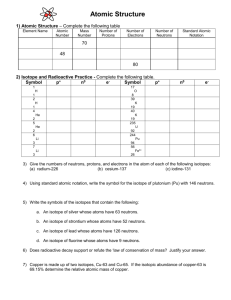
Name: Lab Partner(s): Per: Date: Lab 8: Average Atomic Mass of Halloweenium Chemistry 1 Objective 1. To practice calculating the average atomic mass of an element 2. To gain a better understanding the term percent abundance Introduction In this investigation, you will be determining the average atomic mass of a timely new element, “Halloweenium,” which bears an uncanny resemblance to Halloween candy. Although usual tests on a new element typically require fancy instrumentation, you will be performing more basic tasks, namely counting, massing and calculating. From the average mass and percent abundance of each isotope of Halloweenium, you will calculate the average atomic mass of the element. PROCEDURE 1. 2. 3. 4. 5. 6. Record your bag number in Data Table #1. Remove the candies from the zip-lock bag. Sort the candies by type and record the name of each different isotope (type of candy). Count the number of pieces of each type of candy and record in column # 2. Add up the numbers in column #2 to determine the total number of pieces of candy in the bag. Calculate the percent abundance of each isotope by dividing the number of pieces of that candy by the total number of pieces of candy in the bag. Record. 7. Using the triple beam balance, determine the total mass of each “isotope” by massing all of the “atoms” of the isotope together. Record. 8. Calculate the average mass of each isotope by dividing the total mass by the number of pieces of candy (Column 4/Column 2); record. 9. Replace the candies in the bag and return the candies and the balance to the area designated by your teacher. Clean your work area and wash your hands before you leave the laboratory. DATA - Average Atomic Mass of Halloweenium Table 1. Halloweenium Data Bag # 1. Isotope (candy type) Totals 2. # Atoms of Isotope 3. Percent abundance (Round to 2 decimal places) 4. Total Mass of Isotope, g 5. Average Mass of Isotope, g (Round to 2 decimal places) NA Calculation: Using the percent abundance and average mass of each isotope, calculate the (weighted) average atomic mass of the element “Halloweenium”. 1 Synthesis Show your work! 1. Explain how you determined the percent abundance of each isotopic form of Halloweenium? 2. Why must you calculate the “average” mass of each isotope before calculating the average atomic mass of the element? 3. What is the difference between the atomic number and the mass number of an element? Can atoms of two different elements have the same atomic number? Can they have the same mass number? Why or why not? 4. The four isotopes of lead are listed below, each with its percent abundance. Calculate the average atomic mass of lead. a. Isotope #1 - 82 protons, 122 neutrons - 1.37% b. Isotope #2 - 82 protons, 124 neutrons - 26.26% c. Isotope #3 - 82 protons, 125 neutrons - 20.82% d. Isotope #4 - 82 protons, 126 neutrons - 51.55% 5. Since the average atomic mass of hydrogen is 1.0079 and hydrogen is composed of one proton and one electron then why isn’t the average atomic mass of Helium exactly 4.0316 (4 × 1.0079)? Helium has an average atomic mass of 4.0026. 2