11 CHAPTER 2 PHASE BEHAVIOR OF NATURAL GAS SYSTEMS
advertisement

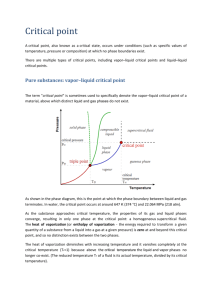
CHAPTER 2 PHASE BEHAVIOR OF NATURAL GAS SYSTEMS The term "natural gas" is used to denote either a mixture of volatile hydrocarbons or a gas phase. When one speaks of "liquefied natural gas"; the term "natural gas" refers to the constituents in the mixture; when one speaks of a "gas cap" in a reservoir, the reference is to a gas phase. In this gas phase, under high pressure, there may be several constituents which, as pure substances, occur as high-boiling liquids. 2.1 PURE SUBSTANCES-VAPOR PRESSURE Pure substances may occur in vapor, liquid, and solid phases depending upon the temperature and pressure. When a substance is in a single phase, its temperature T and pressure P define the volume V of the component. Since three-dimensional figures are more difficult to use than two, pressuretemperature projections of the intersections of the surfaces (Figure 2.1) and pressure-volume sections 1 at constant temperature (Figure 2.2) are more commonly employed . The vapor pressure curve giving the boiling point at various pressures is a most important property of pure substances. Pure hydrocarbons are encountered in processing operations or in the laboratory, and vapor pressure curves become very useful in handling them. Figure 2.3 gives the vapor pressure of the volatile hydrocarbons encountered in the gas industry. 2.2 MIXTURES-RETROGRADE PHENOMENA Mixtures bring an added dimension to phase behavior, as demonstrated by the methane-ethane system 1 (Figure 2.4) . The two-phase region for all possible mixtures of methane and ethane is bounded by the vapor pressure curve for methane, Curve 1, the vapor pressure curve for ethane, Curve 10, and the critical locus connecting the critical points of the pure substances. At pressures above the critical locus all mixtures of methane and ethane remain in single phase. Mixtures 2 to 9 each have a phase boundary curve as shown. The P-T diagram of Figure 2.5 will be used to illustrate the language used in describing the behavior of a mixture. Inside the curve ABCDIE, the mixture is in two phases, vapor and liquid. Outside the border curve, only one phase occurs. The mixture at K is in one phase, and it remains in a single phase with a pressure reduction at constant temperature until point B is reached. Here, a bubble of vapor appears at equilibrium; point B is called a bubble point. Further reduction in pressure causes more vapor to form and, at F, 20 volume % is in the vapor state. Points along the curve ABC are bubble points for the mixture. The mixture at J is in a single phase, and it continues as such with pressure increase until point E is reached. Here, a droplet of liquid or dew appears at equilibrium; the name "dew point" is given to this condition. As further pressure increase occurs from point E, more liquid is condensed as indicated. Likewise, a pressure reduction from L to D will cause dew to form. A pressure reduction on the fluid from M to C causes the single-phase fluid to change abruptly to approximately 50 percent vapor and 50 percent liquid at C. It so happens that the properties of the vapor along the dew-point curve and those of the liquid along the bubble-point curve merge at C, and this is the critical point for the mixture. Compressing pure substances in the vapor state to the vapor pressure curve will cause dew to form; therefore, the behavior from J to E is to be expected. However, the type of phase behavior from L to D 11 to G - the formation of liquid by pressure reduction on a gas - is a reverse phenomenon that of pure substances. The phenomenon of liquid formation by isothermal expansion of single-phase fluid is retrograde condensation. The expansion from D towards G yields a maximum percentage of liquid at G, and the liquid vaporizes in a manner as the pressure is reduced still further from G to E. Graphical representation of the mixtures with more than two components is very complex. Ternary 1 diagrams for methane-propane-n pentane mixture is shown in Figure 2.6 . REFERENCE 1. Katz, D.L., and R.L.Lee, Natural Gas Engineering, Production and Storage, McGraw-Hill Publishing Co., New York (1990). Figure 2.1 Phase diagram for a pure substance. Figure 2.2 Typical pressure-volume diagram for a pure substance. 12 1 Figure 2.3 Vapor pressures of hydrocarbons . 1 Figure 2.4 Phase diagram of methane-ethane mixture . 13 1 Figure 2.5 Phase diagram for a mixture . 1 Figure 2.6 Critical loci for methane-propane-n pentane mixture . 14