Pre-lab 5: Synthesis and TLC of Aspirin
advertisement

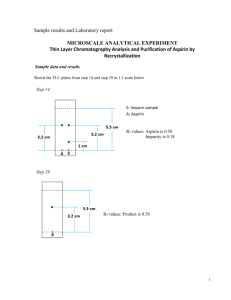
Name:______________ Section:_____________ Pre-lab 5: Synthesis and TLC of Aspirin Read the background information and answer the following question before lab. Today you will need to calculate the % yield of your aspirin, so the pre-lab will set you up for this. 1. What are the molar masses of salicylic acid (C7O3H6), acetic anhydride (C4O3H6), and acetylsalicylic acid (C9O4H8)? (Hint: Chapter 6.5 page 226) 2. If 1g of salicylic acid is used to make acetylsalicylic acid, how much acetylsalicylic acid, in grams, is made? The reaction of salicylic acid to make acetylsalicylic acid is 1mol to 1mol. (Hint: use molar masses from #1 to go from grams to mol and Chapter 6.6 -6.7, page 230) 3. If 2mL of acetic anhydride is used to make acetylsalicylic acid, how much acetylsalicylic acid, in grams, is made? The reaction of acetic anhydride to make acetylsalicylic acid is 1mol to 1mol. (Hint: use the density of acetic acid (density =1.082 g/mL) to find grams of acetic acid.) ~ 62 ~ 4. Which of the answers for #2 and #3 is a lower value? The reactant that produced this value is called the limiting reagent. Is the limiting reagent salicylic acid or acetic anhydride? 5. The lower value in #4 is also called the theoretical yield of the reaction. If your experimental yield was 0.98g, what is the % yield for your experiment? (Hint: Chapter 6.8, page 236, %yield = (actual yield/theoretical yield) x 100%) ~ 63 ~ Lab 5: Synthesis and TLC of Aspirin Objective: The objectives of this experiment are to make aspirin and then use TLC to determine the purity of your aspirin. Background Information: Synthesis of Aspirin Salicylic acid was once used as a pain killer, but it caused irritation to the stomach lining. Scientist found that by adding a new functional group to salicylic acid and forming acetylsalicylic acid, the stomach irritation was reduced. The reaction for this is shown in Figure 1. salicylic acid acetic anhydride acetylsalicylic acid acetic acid Figure 1. Over time Acetylsalicylic acid (aspirin) will breakdown into salicylic acid and acetic acid. In order to slow this breakdown aspirin should be stored in a dry place. Today you will look at aspirin that has been left in water for a little over a week and has broken down. You can tell when aspirin has broken down by the vinegar smell of acetic acid. Since acetic acid is also produced by the aspirin reaction, if the aspirin made today is not rinsed well, acetic acid will also show up in the aspirin sample. TLC Analysis TLC stands for Thin Layer Chromatography. This is a technique used to look at samples that may contain multiple compounds in them or determine an unknown when you have multiple known samples to compare it to. You will be using TLC to compare the aspirin that you make to a commercial aspirin, the starting material (salicylic acid), and a decomposed sample of aspirin. ~ 64 ~ Procedure: Data: Synthesis of Aspirin Equipment list: Small Vial with Lid Vial Holder Thermometer Ring Stand Thermometer Clamp Ice Bath A) Synthesis of Aspirin 1) Place the small vial on the balance and tare it. 2) Weigh out about 1g of salicylic acid into the vial. 3) Record the exact weight on the blank line. Include units. 4) Record this value on the report sheet also. 5) Add 2 mL of acetic anhydride to the vial. 6) Add 0.4 mL of sulfuric acid (catalyst) to the vial. 7) Mix the contents of the vial by gently swirling. 8) Place a thermometer in the vial. 9) Place the vial in the vial holder. Support the thermometer. 10) Monitor the temperature of the reaction. 11) When the temperature begins to decrease, make an ice bath. __________________ B) Set up an Ice Bath 1) Place the vial in a 250 mL beaker. 3) Pack enough ice around the vial to cover the liquid in the vial. 4) The acetylsalicylic acid should begin to form as a solid in the vial. 5) Add 2 mL of water to the vial. 6) Filter and dry the aspirin using a Buchner funnel. C) Recrystalize the Aspirin (if time permits) 1) Dissolve your aspirin in hot toluene. 2) Allow to cool in an ice bath. 3) Filter and dry the aspirin again using a Buchner funnel. 7) Weigh the aspirin. 8) Record the weight on the blank line. Include units. 9) Record the weight on your report sheet also. ~ 65 ~ __________________ 10) a. What is the theoretical yield for the acetylsalicylic acid? report sheet. Record the final value on the b. What is the % yield for your acetylsalicylic acid? Record the final value on the report sheet. TLC Analysis of Aspirin Equipment list: Three- 50 mL Erlenmeyer Flasks Small Tubes TLC Chamber TLC Plate Ruler Pencil D) Set-up 1) Place a small amount of salicylic acid, commercial aspirin, and synthesis aspirin in three separate, labeled 50 mL Erlenmeyer flasks. 2) Add 5 mL of ethanol and mix by gently swirling. 3) Wait until the contents of all of the flasks are dissolved. 4) Obtain a TLC plate by handling the edges and not the front. 5) With a pencil, mark 1cm and 9cm up the plate and draw a line. 6) Make 4 marks on the 1 cm line, 1 for each sample. ~ 66 ~ 7) Step 5 and 6 are shown in Figure 2. 8) Label on Figure 2, where each sample will go on the plate. Step 5 Step 6 9cm 1cm Figure 2. 9) Place each sample you labeled on the Figure on the real TLC plate on the marks, using the small tubes. 10) The line 9 cm up the plate will be the stop point. 11) Use the UV light to check that your dots works. E) Running the TLC Platess 1) Put 15 mL of the TLC solution in the TLC chamber. 2) Carefully place the TLC plate in the chamber. 3) Put the lid on the chamber. 4) Watch the solution move up the TLC plate, until it reaches the 9cm line. 5) Remove the TLC plate from the chamber. 6) Allow the plate to dry completely. 7) Use the UV light to look at the spots on the TLC plate. 8) Draw the location and label the color of the dots on the TLC plate drawing below. 9) What did your aspirin look like? Compare it to the other samples on the TLC plate. ~ 67 ~ Name:_______________________ Lab 5: Synthesis and TLC of Aspirin Report Sheet Partner(s):____________________ _____________________________ Section:______________________ Synthesis of Aspirin A.3. Weight of the salicylic acid._____________________ C.8. Weight of the aspirin.__________________ C.10.a. Theoretical yield for the acetylsalicylic acid.________________ C.10.b. % yield for your acetylsalicylic acid._________________ TLC Analysis of Aspirin Redraw the dots and label the colors from E.8. ~ 68 ~ This page was intentionally left blank!! ~ 69 ~