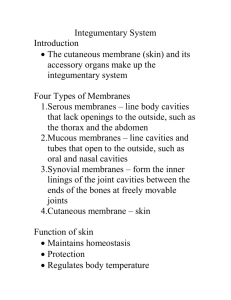
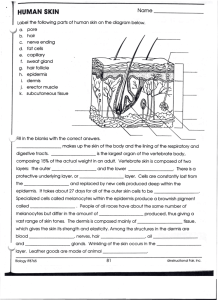
In vitro mechanical characterization of human skin layers
advertisement

In vitro mechanical characterization of human skin layers: stratum corneum, epidermis and hypodermis. ii Individual skin layer mechanics: Stratum corneum, epidermis, and hypodermis PROEFSCHRIFT ter verkrijging van de graad van doctor aan de Technische Universiteit Eindhoven, op gezag van de Rector Magnificus, prof.dr.ir. C.J. van Duijn, voor een commissie aangewezen door het College voor Promoties in het openbaar te verdedigen op donderdag 21 januari 2006 om 16.00 uur door Marion Geerligs geboren te Hoogezand-Sappemeer iv Dit proefschrift (De documentatie van het proefontwerp) is goedgekeurd door de promotoren: prof.dr.ir. F.P.T. Baaijens Copromotoren: dr.ir. C.W.J. Oomens en dr.ir. G.W.M. Peters Contents Summary .............................................................................................. viii 1 General introduction........................................................................... 1 1.1 Introduction............................................................................................................... 2 1.2 A mechanical view of skin anatomy and physiology ............................................... 4 1.2.1 Skin relief........................................................................................................... 5 1.2.2 Stratum corneum................................................................................................ 5 1.2.3 Viable epidermis ................................................................................................ 6 1.2.4 Dermal-epidermal junction ................................................................................ 8 1.2.5 Dermis................................................................................................................ 8 1.2.6 Hypodermis........................................................................................................ 8 1.3 State-of-the-art in skin layer mechanics ................................................................. 10 1.3.1 In vivo vs in vitro experiments ........................................................................ 10 1.3.2 Mechanical behavior of the stratum corneum ................................................. 10 1.3.3 Mechanical behavior of the viable epidermis .................................................. 12 1.3.4 Hypodermis...................................................................................................... 12 1.4 Aim and Outline ..................................................................................................... 13 2 Isolation and preservation methods for the epidermis and stratum corneum ............................................................................................. 15 2.1 Introduction............................................................................................................. 16 2.2 Skin preparation and analyses ................................................................................ 18 2.2.1 Skin preparation.......................................................................................................... 18 2.2.2 Histological examination ............................................................................................ 18 2.2.3 Analyses of skin viability ........................................................................................... 19 2.3 Epidermal isolation techniques ............................................................................... 19 2.3.1 Mechanical separation ................................................................................................ 19 2.3.2 Ionic change................................................................................................................ 20 2.3.3 Heat............................................................................................................................. 21 2.3.4 Enzymatic digestion ................................................................................................... 22 2.3.5 Microwave irradiation ................................................................................................ 23 2.4 Isolation techniques for the stratum corneum......................................................... 24 2.4.1 Mechanical separation ................................................................................................ 24 vi 2.4.2 Chemical separation ................................................................................................... 25 2.4.3 Enzymatic digestion ................................................................................................... 26 2.5 Preservation of the upper skin layers ...................................................................... 27 2.5.1 Short-term storage ...................................................................................................... 27 2.5.2 Long-term storage ...................................................................................................... 28 2.6 Discussion ............................................................................................................... 30 3 Linear shear response of the upper skin layers ............................. 35 3.1 Introduction............................................................................................................. 36 3.2 Methods .................................................................................................................. 37 3.2.1 Sample preparation .......................................................................................... 37 3.2.2 Experimental set-up ......................................................................................... 38 3.2.3 Rheological methods ....................................................................................... 41 3.2.4 Experimental procedures ................................................................................. 42 3.2.5 Histological examination ................................................................................. 43 3.3 Results..................................................................................................................... 44 3.4 Discussion ............................................................................................................... 48 4 A new indentation method to determine the mechanical properties of epidermis ....................................................................................... 51 4.1 Introduction............................................................................................................. 52 4.1.1 Sample preparation .......................................................................................... 53 4.1.2 Experimental procedure ................................................................................... 54 4.1.3 Determination of the Young‟s modulus .......................................................... 55 4.2 Results..................................................................................................................... 57 4.3 Discussion ............................................................................................................... 59 5 Linear viscoelastic behavior of subcutaneous adipose tissue ....... 63 5.1 Introduction............................................................................................................. 64 5.2 Methods and Materials ........................................................................................... 66 5.2.1 Sample preparation .......................................................................................... 66 5.2.2 Rheological methods ....................................................................................... 66 5.2.3 Testing procedure ............................................................................................ 68 5.2.4 Statistics ........................................................................................................... 69 5.3 Results..................................................................................................................... 69 5.3.1 Small oscillatory strain behavior ..................................................................... 69 5.3.2 Model application ............................................................................................ 70 5.3.3 Time-Temperature Superposition .................................................................... 71 5.3.4 Freezing effects................................................................................................ 72 5.4 Discussion ............................................................................................................... 73 6 Does subcutaneous adipose tissue behave as an (anti-)thyxotropic material? ............................................................................................ 77 6.1 Introduction............................................................................................................. 78 6.2 Materials & Methods .............................................................................................. 79 6.2.1 Sample preparation ..................................................................................................... 79 6.2.2 Rheological methods .................................................................................................. 80 6.3 Results..................................................................................................................... 82 6.3.1 Long term small strain behavior ................................................................................. 82 6.3.2 Large strain experiments ............................................................................................ 83 6.4 Discussion ............................................................................................................... 85 7 General discussion............................................................................. 89 7.1 Introductory remarks .............................................................................................. 90 7.2 In vitro model ......................................................................................................... 91 7.3 Mechanical methods ............................................................................................... 92 7.4 Main findings .......................................................................................................... 94 7.4.1 Small strain behavior of the epidermal layers ............................................................ 94 7.4.2 Mechanical behavior of the subcutaneous adipose tissue .......................................... 95 7.5 Implications for clinical and cosmetic applications ............................................... 95 7.6 Recommendations................................................................................................... 96 7.7 General conclusion ................................................................................................. 97 Samenvatting ........................................................................................ 99 Dankwoord.......................................................................................... 101 Curriculum Vitae ............................................................................... 103 References ........................................................................................... 104 Summary In vitro mechanical characterization of human skin layers Stratum corneum, epidermis, and hypodermis The human skin is composed of several layers, each with an unique structure and function. Knowledge about the mechanical behavior of these skin layers is important for clinical and cosmetic research, such as the development of personal care products and the understanding of skin diseases. Until today, most research studies were performed in vivo and focused on the mid-layer, the dermis. However, clinical and cosmetic applications require more detailed knowledge about the skin layers at the skin surface, viable epidermis and stratum corneum, and the deeper lying hypodermis. Studying these layers in an in vivo set up is much more challenging. The different length scales, ranging from μm for the stratum corneum to cm for the hypodermis, the interwoven layered structure and the inverse relation between penetration depth and resolution of noninvasive measurement techniques form major problems. As a consequence, hardly any data are available for the viable epidermis and hypodermis and reported data for stratum corneum are inconsistent. The objective of this thesis was therefore to characterize the mechanical behavior of individual skin layers in vitro and, for that, to develop the required experimental procedures. It was considered essential to perform experiments with samples of consistent quality in an accurate measurement set-up in a well-controlled environment. To obtain samples of consistent quality, the integrity and viability of a skin layer needs to be maintained. Therefore, various isolation and preservation methods were investigated on tissue performance, reproducibility, and ease of handling. Because of the inhomogeneous layered structure of the upper skin layers, mechanical properties of the stratum corneum and viable epidermis were determined for various loading directions. First, the stratum corneum and epidermis were subjected to shear over a wide frequency range and with varying temperature and humidity. The typical geometry of the upper skin layers required preliminary testing series in order to define right experimental conditions to ensure reliable results. Subsequently, micro-indentation experiments were applied using a spherical tip with a relatively large diameter. The Young‟s moduli was derived via an analytical and numerical method. Because of the complexity of measuring those skin layers, it was decided to focus on small deformations first. For both types of loading, result were highly reproducible. The shear tests demonstrated that the shear modulus is influenced by humidity but not by temperature in the measured range. The indentation tests showed that analytical methods are not appropriate to assess the Young‟s modulus, such that finite element are required. If the skin is loaded perpendicularly, the stiffness of the epidermis and stratum corneum, which is about 1-2 MPa, is about a factor 100 higher than for shear. No significant differences in stiffness between the stratum corneum and viable epidermis were observed per loading type. The results of these tests prove that it is essential to taken into account the highly anisotropy of the tissue in numerical models. Rheological methods were developed to study the mechanical response of the subcutaneous adipose tissue. In the small linear viscoelastic strain regime, the shear modulus showed a frequency- and temperature-dependent behavior and is about 7.5 kPa at 10 rad/s and 37°C. Time-Temperature Superposition is applicable through shifting the shear modulus horizontally. A power-law function model was able to describe the frequency dependent behavior at constant temperature as well as the measured stress relaxation behavior. Prolonged loading of small strain results into a dramatic stiffening of the material. Loading-unloading cycles showed that this behavior is reversible. In addition, various large strain history sequences showed that stress-strain responses are reproducible up to 0.15 strain. When the strain further increases, the stress is decreasing for subsequent loading cycles and, above 0.3 strain, the stress response become stationary. These contrary results due to time effects and strain effects indicate that adipose tissue likely behaves as an (anti-)thixotropic material, meaning that a constitutive model should contain parameters to describe the build-up and breakdown of material structure. However, further experimental research is needed to fully understand the thixotropic behavior before such a model can be worked out in detail. In conclusion, this thesis evaluates the mechanical behavior of stratum corneum, epidermis and hypodermis using various in vitro set-ups. It was proven that for all skin layers reproducible results can be obtained. The research was aimed at developing reliable methods to determine the mechanical behavior of individual human skin layers. Future work should be focused on the relationship between tissue deformation using imaging techniques and heading to the determination of the skin‟s failure behavior in relation to clinical and cosmetic treatments. Chapter 1 General introduction 2 Chapter 1 1.1 Introduction The largest organ of the human body, the skin, has a major role in providing a barrier against the hostile external environment. The skin prevents excessive water loss from the aqueous interior, the ingress of foreign chemicals and micro-organisms and provides strength and resistance to mechanical loading. Other functions include insulation, temperature regulation and sensation. To fulfill these functions, mechanical stability is as important as mechanical flexibility. However, the mechanical balance of skin can be threatened by diseases or medical or cosmetic treatments. In order to understand the skin behavior during treatments or diseases, knowledge of the mechanical behavior of healthy skin in normal conditions is essential. Human skin is composed of several layers, each with a unique structure and function, but most research on its mechanical properties have ignored this non-uniform layered structure. For many clinical and cosmetic applications, however, knowledge of the mechanical behavior of the various skin layers is indispensible (Figure 1.1). For example, the benefit of transdermal drug delivery is that the microneedles exclusively damage the pain-free outer skin layer, the epidermis. Its mechanical response is therefore of particular interest. For needle insertion procedures going deeper into the skin or diseases such as pressure ulcers, the mechanical properties of all individual skin layers play a role. Although often not recognized, this is also the case during skin adhesive removal or the use of consumer products such as shavers. For all these applications, the subcutaneous fat layer considerably contributes by attenuating or dispersing the external pressures, even when those are very small [1]. In addition, mechanical properties of the distinct skin layers are needed to grow them artificially, serving a wide application field; artificial outer skin can substitute animal and clinical testing in evaluating drugs, cosmetics and other consumer products, while engineered fatty tissue facilitates large volume soft tissue augmentation in plastic surgery. Furthermore, the mechanical behavior of subcutaneous fat is crucial for many other clinical treatments outside the scope of this thesis, such as liposuction surgery and cellulite treatments. As the top layer of the epidermis, the stratum corneum, is the first barrier between the human body and its environment, it is obvious that the mechanical response of this layer needs to be understood. The significance of a proper understanding of the mechanical behavior of the other part of the epidermis, the viable epidermis, and the subcutaneous fat tissue is not yet commonly felt. Until today, research on skin mechanics has mainly focused on full-thickness skin, the mid-layer (dermis) and the stratum corneum. Hardly any experimental data are available for the other skin layers, i.e. the viable epidermis and the subcuateneous fat. In addition, there is no consistency in data for the stratum corneum. Hence, accurate numerical models including the mechanical behavior of individual skin layers have not yet been developed for any of the applications mentioned above. This thesis therefore focuses on the mechanical characterization of individual skin General introduction 3 (a) (b) (c) (d) (e) (f) Figure 1.1 Clinical and cosmetic applications where the mechanical properties of separate skin layers are important: (a) transdermal drug delivery; (b) skin-device contact such as during shaving; (c) removal of adhesives such as ECG electrodes; (d) decubitus; (e) needle insertion procedures; (f) tissue engineering. 4 Chapter 1 layers. Before the scope and outline of the thesis is given, the anatomy of the skin and the state of the art on skin layer mechanics is shortly discussed. 1.2 A mechanical view of skin anatomy and physiology Mechanical properties of skin are very diverse and depend on body site, age, race and gender. Individual factors like exposure to UV irradiation, the use of creams and the person‟s health and nutritional status may also modify the mechanical properties. From the skin surface inwards, skin is composed of epidermis, dermis and hypodermis (Figure 1.2 ). The epidermis is mainly composed of cells migrating to the skin surface. The stratum corneum is considered as a separate layer because of its specific barrier properties. It consists of non-viable cells and is very firm but pliable and wrinkled. The other part of the epidermis, the viable epidermis, is also wrinkled. The underlying layer, the dermis, is largely composed of a very dense fiber network dominating the mechanical behavior of the total skin. The deepest skin layer, the hypodermis or subcutaneous adipose tissue, is composed of loose fatty connective tissue. All skin layers contain microstructures like blood vessels, lymph vessels, nerve endings, sweat glands and hair follicles. The influence of these structures on the mechanical properties are consideration to be ignorable, because the interest is on the bulk mechanical behavior caused by the main components of the skin layer. Of all skin layers, the dermis is the layer that is studied most. Consequently, data on dermal properties are readily available. This thesis therefore focuses on the mechanical behavior of the other layers, i.e. stratum corneum, viable epidermis and hypodermis. Consequently, the anatomy and physiology of these skin layers are of particular interest. Figure 1.2 Schematic representation of the different skin layers. General introduction 5 1.2.1 Skin relief The relief of the skin surface is formed by the association of furrows, follicular orifices and sweat pores, and slightly protruding corneocytes. On most body sites, the main furrows, called primary lines, are 70-200 μm deep, follow at least two directions and delimit plateaus of variable shapes. The follicular orifices are located in the junction of the furrows, whereas the sweat pores are mainly found on the plateaus or in more superficial furrows, called secondary lines, being 20-70 μm deep. The third type of furrows separate groups of corneocytes. The network of furrows varies with age and gender. The main function of the furrows is considered to be mechanical. By (partially) smoothing out, the skin surface and the epidermis can extend without loading the cells. Their anatomical distribution at a body site reflects the direction of the mechanical constraints sustained by the skin. These furrows cannot be ignored when methods are developed to mechanically characterize the stratum corneum and the epidermis. 1.2.2 Stratum corneum The stratum corneum is composed of corneocytes, which are hexagonal flat cells without a nucleus, held together by lipids and desmosomes in what is commonly referred to as a brick-and-mortar structure (Figure 1.3). The diameter and thickness are ranging from 25 to 45 μm and approximately 0.3-0.7 μm, respectively [2,3]. The stratum corneum consists of 15-25 [3,4] layers of corneocytes, resulting in a total layer thickness of about 10-25 μm [5]. The lipids are arranged in lamellar sheets, which consist of membrane-like bilayers of ceramides, cholesterol, and fatty acids together with small amounts of phospholipids and glucosylceramides. The intercellular spaces, i.e. the distance between neighboring corneocytes, are about 0.1-0.3 μm [6]. Desmosomes, also called corneosomes, are specialized inter-corneocyte linkages formed by proteins and, together with the lipids, they maintain the integrity of the stratum corneum [7]. The lipids form the major permeability barrier to the loss of water from the underlying epidermis. The stratum corneum is continuously renewed. Cells are shed from the outside and replaced by new ones. Changes in structure, composition and function of the corneocytes occur as they move toward the outer skin surface. Cells of the deeper layers of the stratum corneum are thicker and have more densely packed arrays of keratins, a more fragile cornified cell envelope and a greater variety of modifications for cell attachment as compared to cells of the outer stratum corneum. Consequently, the deeper part of the stratum corneum has a major influence on its overall mechanical behavior. The outer stratum corneum cells have less capacity to bind water. The cells in the outermost stratum corneum have a rigid cornified envelope and in the same area, the desmosomes undergo proteolytic degradation. These changes contribute to the continuous shedding of the cells at the surface of the stratum corneum. Renewal time of the stratum corneum and viable epidermis under normal conditions varies from 6 to 30 days [8]. 6 Chapter 1 (a) (b) Figure 1.3: The morphology of the stratum corneum. (a) schematic drawing (b) cryostat section of normal human skin treated with Sorensen’s alkaline buffer and methylene blue to show the brick-and-mortar structure of the stratum corneum. Obtained from Marks [9]. Although the corneocytes are non-viable, the stratum corneum is considered to be fully functional, particularly in terms of barrier properties, and retains metabolic functions [10]. The mechanical properties of both stratum corneum and viable epidermis are influenced by environmental conditions such as relative humidity (RH) and temperature. In addition, topical application either of pure water, moisturizers or emollients changes the hydration state of the stratum corneum, significantly modifying some of its mechanical properties. Although the hydration level depends on those factors, the hydration in the stratum corneum under normal conditions varies from 5-10% near the surface up to 30% near the transition to the viable epidermis. Bound water as component of proteins and lipids, accounts for 20-30% of the total water volume. The total water content varies little between 30% and 60% RH but considerably increases thereafter [11]. When fully hydrated, the stratum corneum is able to become as twice as thick. In an in vitro situation, however, the stratum corneum can increase to 400% of its original thickness [10]. The stratum corneum matches the creases forming the skin surface. The deeper the furrows and the steeper their sides, the higher their physiological range of extension. The direction of the higher extensibility is perpendicular to the direction of the main furrows. As a consequence, the stratum corneum in vivo hardly experience elongation stresses, but only unfolding. This unfolding is an important feature of the overall skin resistance to stretching. 1.2.3 Viable epidermis The viable epidermis is a layered structure, consisting of three layers or „strata‟. The bulk of epidermal cells are the keratinocytes, which migrate to the skin surface and become non-viable in the stratum corneum. Other cell types within the viable epidermis include melanocytes, Langerhans cells and Merkel cells. General introduction 7 Keratinocytes change their shape, size and physical properties when migrating to the skin surface. The structure of an individual keratinocyte correlates with its position within the epidermis and its state of differentiation, which is reflected by the different strata: the stratum basale, the stratum spinosum and the stratum granulosum (Figure 1.4). The deepest layer is the stratum basale in which cell division occurs. It consists of 1 to 3 layers of small cubic cells. As the cells move towards the surface, they become larger and polyhedral. The next layer is the stratum spinosum. The keratinocytes have become polyhedral and are connected by desmosomes, which are symmetrical laminated structures. The shape of the polyhedral cells becomes more flattened as they move further outward. In the uppermost layers of the stratum spinosum so-called lamellar granules appear. Those lamellar granules are lipid-synthesizing organelles that migrate toward the periphery of the cell and eventually become extruded into the intercellular compartment in the next layer, the stratum granulosum. At this stage of differentiation, the degradation of mitochondria and nuclei starts and the cytoplasm of the flattened cells become almost filled by keratohyalin masses and filaments. Furthermore, the cell membrane becomes gradually thicker. The thickness of the viable epidermis varies roughly from 30-100 μm [12]. The number of cell layers differs from 5 up to 10. The cells are communicating by very strong desmosomes in the very compact tissue; the intercellular spaces occupy less than 2% of the volume [5,13]. Consequently, the viable epidermis is considered to be more rigid than other soft tissues. Because of its non-vascular structure, the epidermal cells are nourished from plasma that originates in the dermal blood vessels and then transits through the epidermal-dermal junction. stratum corneum granulous layer LG spinous layer N KF D basal layer Figure 1.4: A schematic drawing and histological cross-section to show the structure of the epidermis. In the schematic drawing the nucleus (N), the keratin filaments (KF), the desmosomes (D) and the lamellar granules (LG) are depicted. The histological section is taken from the skin of a young woman, obtained from Montagna et al. [14]. 8 Chapter 1 1.2.4 Dermal-epidermal junction The boundary between the dermis and epidermis is called the dermal-epidermal junction, which provides a physical barrier for cells and large molecules. Four distinctive zones in this strong junction can be identified: 1) the plasma membrane and hemidesmosomes of the basal keratinocytes adhered to the junction, 2) the lamina lucida zone with anchoring filaments, 3) the lamina densa, and 4) the amorphous sublamina densa fibrillar zone (see also Figure 2.1). The firmness of the attachment is enhanced by parts of the epidermis penetrating the papillary dermis resulting in large cones called rete ridges or papillae [15]. The major point of weakness is considered to be the lamina lucida [16]. The dermal-epidermal junction length over a straight line ranges from 1.1 to 1.3 [5]. 1.2.5 Dermis The dermis can be divided into two anatomical regions: the papillary and reticular dermis. The papillary dermis is the thinner outermost portion of the dermis, constituting approximately 10% of the 1-4 mm thick dermis. It contains smaller and more loosely distributed elastic and collagen fibrils within a greater amount of substance than the underlying reticular dermis. Its content in water and vascular volume show physiological variations that can alter the mechanical behavior of skin as a whole. In addition, collagen and elastin fibers are mostly vertically oriented in the papillary region and connect to the dermal-epidermal junction, whereas in the reticular dermis they are horizontally oriented. The amorphous ground substance acts as a viscous gel-like material, which does not leak out of the dermis, even under high pressure. The reticular dermis forms a solid structure with a permanent tension. The dermis has a mainly mechanical function. The reticular dermis is able to extend up to about 25% by undulating the collagen fibers, whereas it can be squeezed due to the capacity to displace the ground substance laterally. The elastic fiber network ensures full recovery of tissue shape and architecture after deformation. The permanent tension in the reticular dermis generates the folding of the nonelastic overlying structures and hence, the skin surface relief. The fiber network in the papillary dermis contributes to the protection of vessels and cells against mechanical insults. The dermis nourishes the epidermis. In the papillary dermis, therefore, the microvasculature consists of papillary loops exchanging with extravascular elements and a horizontal plexus in which the loops emerge. Although the vascularization throughout the dermis seems sparse, the supply of the papillary loops is ensured by arterioles irrigated from the deep dermis. 1.2.6 Hypodermis The hypodermis is defined as the adipose tissue layer found between the dermis and the aponeurosis and fasciae of the muscles. Its thickness varies with anatomical site, age, sex, race, endocrine and nutritional status of the individual. The subcutaneous adipose General introduction 9 tissue is structurally and functionally well integrated with the dermis through nerve and vascular networks and the continuity of epidermal appendages such as hairs and nerve endings. The bulk of subcutaneous adipose tissue is a loose association of lipid-filled cells called white adipocytes, which are held in a framework of collagen fibers. Only one third of adipose tissue contains mature adipocytes [17]. In addition to the adipocytes, the remaining two third contains stromal-vascular cells including fibroblastic connective tissue cells, leukocytes, macrophages, and pre-adipocytes [18]. Adipose tissue has little extracellular matrix compared to other connective tissues. Stored fat is the predominant component of the adipocytes: the size of the lipid droplet can exceed 50 μm. The cytoplasm and nucleus appears as a thin rim at the periphery of the cell (Figure 1.5). The diameter of the entire white adipocyte is variable, ranging between 30 and 70 μm [17]. Collections of white adipocytes comprise fat lobules, each of which is supplied by an arteriole and surrounded by connective tissue septae. Each adipocyte is in contact with at least one capillary. The good blood supply is necessary for the exchange of metabolites and allows the adipocytes to function effectively. The subcutaneous adipose tissue of the lower trunk and the gluteal thigh region has a thin fascial plane dividing it into superficial and deep portions. Morphological differences are observed between these two adipose tissue layers [19]. N A W A V (a) (b) Figure 1.5: Schematic drawing and histological section of subcutaneous adipose tissue showing white adipocytes (WA) with the nucleus (N) at the periphery. The adipocytes are in good contact with the blood circulation via the arterioles (not visible here) which branches the larger arteries (A) and veins (V). The mechanical function of the subcutaneous adipose tissue has a double purpose: first to allow the overlying skin to move as a whole, both horizontally and vertically, and second, to attenuate and disperse spells of external pressure. 10 Chapter 1 1.3 State-of-the-art in skin layer mechanics Measurement methods and mechanical properties of skin have already been extensively reviewed in the literature [5,20,21]. Therefore, given the focus of this thesis, this review is limited to studies on the behavior of stratum corneum, viable epidermis and hypodermis. More specifically, mainly force-elongation studies, either in vivo or in vitro, and currently available constitutive models are discussed. 1.3.1 In vivo vs in vitro experiments When measurements regarding skin mechanics are carried out in vivo, the human skin has its natural pre-stress and skin relief. The number of in vivo measurement methods is, however, limited [21] and a numerical-experimental approach is usually required. In any in vivo study, it is difficult to determine the contribution of each individual skin layer to the overall skin response, whereas in vitro measurement methods offer the potential to perform well-controlled experiments on individual skin layers. Another benefit is that all kinds of mechanical testing can be applied and a wide range of reliable direct measurement methods becomes available. However, due to the limited availability of skin grafts, the number of experiments, the variety of skins, and the variety of body sites might be limited. The appropriateness of in vitro experiments on the stratum corneum should be carefully considered. In vivo, the stratum corneum partly unfolds when the total skin is stretched, but does not elongate. Therefore, in vitro mechanical characterization is only relevant to the mechanical function of the skin under normal in vivo conditions, when its hardness and capacity to absorb mechanical shocks are of concern. Stretching out of the stratum corneum exclusively occurs in critical, extra-physiological situations. 1.3.2 Mechanical behavior of the stratum corneum Force-elongation curves at constant elongation rate show one, two or three phases depending on the hydration level in an in vitro situation (Figure 1.6) [22]. The first phase, up to a 10% extension, is considered to be purely elastic. The next phase, absent in low RH, is an irreversible elongation with a low slope, with strains ranging from 20125%. Only almost fully hydrated stratum corneum show the last phase before rupture, where strain hardening is observed before rupture, at approximately 200% extension. The slope becomes steeper if the extension rate rises, confirming the viscoelastic nature of the material.Although the corneocytes are very elongated in tensile testing, the final rupture is always extracellular and most likely at the desmosomes [8]. From the 1970s, various authors performed tensile tests [22,23,8,24,25,26]. Thereafter, torsional techniques were developed to measure the stratum corneum behavior in vivo [27,28,29,30]. From the nineties, nano-indentation techniques have been introduced to determine the Young‟s modulus in vitro [31,32], whereas an in vivo indentation technique had been developed already some years before [33]. Furthermore, imaging General introduction 11 Load [g] 40 30 in vivo range techniques such as ultrasound and magnetic resonance elastography have been used to estimate mechanical properties [32,21]. 32% RH 76% RH 20 98% RH 10 I II III 30 Elongation [%] 120 Figure 1.6 Typical force elongation curves for the stratum corneum at different RH showing different phases: the elastic phase (I), the plastic phase (II) and the strain hardening phase (III). Obtained from [22]. Reported Young‟s moduli vary over more than three decades, from a few MPa to GPa [34,24,35,23]. The range of tensile moduli for various RH is shown in Figure 1.7. As indicated in this figure, the stiffness of the stratum corneum varies from rubber-like to nylon-like over the RH range. The differences may be due to a combination of several reasons, such as regional differences, anisotropy, differences between species but also test conditions such as sample preparation, difficulties in determining sample dimensions and controlling the environmental conditions. A general trend, however, is a more pronounced decrease of the elastic modulus beyond 60% RH. At a constant RH, the stratum corneum hydration increases by 50% when the temperature rises from 20°C to 30°C. At higher RH, however, the temperature dependence of the modulus decreases and declines to a minimum above 90% RH. More common trends due to an increase in RH or temperature include an increase of the maximum extension and rupture work, and a reduction of the force at rupture [22,24,36]. Preconditioning effects does not exist with stratum corneum, which is an important difference with the whole skin, indicating the absence of mobile components in the material [35]. In the same study, it was shown that stratum corneum behaves isotropically in transversal plane only. Current constitutive models of the stratum corneum are based on traction, relaxation and creep tests [5]. From the tests, it was concluded that the model should include elasticity, 12 Chapter 1 non-linear viscosity and strain hardening parameters. The link between the defined parameters and the anatomical components is yet to be determined. Figure 1.7 An overview of Young’s moduli as function of the RH derived from in vitro tensile tests on stratum corneum. 1.3.3 Mechanical behavior of the viable epidermis Only recently, few studies have focused on this part of the epidermis. From an indentation study, a local Young‟s modulus for the viable epidermis of murine ear skin has been reported to be a few MPa [37,38]. However, murine skin has more dense hair, a higher hair follicle density and a very thin epidermis compared to human skin. A combined experimental-numerical approach on in vivo human skin led to an estimated value of about 0.5 kPa for the Young‟s modulus of the upper human skin layers including the papillar dermis [38,1]. The authors hypothesized that this low value is due to the fact that the influence of the stratum corneum is negligble on the overall mechanical response of the skin when suction is performed with small aperture sizes. Because experimental data is limited, a constitutive model describing the mechanical behavior of viable epidermis is not yet available. 1.3.4 Hypodermis A limited number of studies is available regarding the mechanical behavior of subcutaneous adipose tissue, either applying shear [39], compression [40,39], indentation [41,42] or suction [38,1,39,43,40]. Only the suction experiments were performed in vitro. Measured Young‟s moduli vary from a few kPa up to more than 100 kPa. All studies give limited descriptions of the mechanical behavior as they were developed for very specific applications. Consequently, a proper constitutive model based on experimental data is not available yet. Current models are either limited to small strain behavior [38,44] or based on other soft connective tissues. General introduction 13 1.4 Aim and Outline The objective of this thesis is to characterize the mechanical behavior of individual skin layers in vitro and, for that, to develop the required experimental procedures. The focus is on those skin layers for which hardly any data are available, i.e. the viable epidermis and hypodermis, and those with inconsistent data, i.e. the stratum corneum. The results should provide insight into the relationship between the mechanical responses of the various skin layers to their structure and, hence, provide better understanding of the way a treatment or disease affects the skin. Furthermore, the experimental data should be suitable as input for constitutive models. Previous studies, such as the various in vitro tensile tests on the stratum corneum, have indicated that differences in mechanical properties of the epidermis and stratum corneum cannot be caused by variations in humidity and temperature only, but also by test conditions, anisotropy, sample preparation, and so on. It is therefore essential to perform experiments with samples of consistent quality in an accurate measurement set-up in a well-controlled environment. This will be initially done for relatively simple small strain experiments in various directions under different environmental conditions. If this small strain behavior is reproducible and well-understood, it has become meaningful to explore the non-linear behavior. In order to obtain in vitro samples of consistent quality, various isolation and preservation treatments are first thoroughly investigated for both skin layers (Chapter 2). Subsequently, a rheological measurement set-up has been designed to measure the shear response of thin, soft tissues in a controlled environment (Chapter 3). A microindentation method has been adapted to enable the measurement of loading perpendicular to the skin surface (Chapter 4). Because viable epidermis cannot be isolated as a single layer, a numerical model is introduced to derive its properties from the shear and indentation experiments on stratum corneum and whole epidermis. Rheological methods are developed to study the shear response of subcutaneous adipose tissue (Chapter 5). In order to study the large deformation behavior, it is essential to understand its small strain behavior first. From those results, a constitutive model describing the linear viscoelastic behavior of subcutaneous adipose tissue has been built. Then, a set of experiments were designed to study the large deformation behavior and, in relation to that, the time-dependent behavior (Chapter 6). Finally (Chapter 7), a general discussion contemplates the chosen measurement methods for the skin layers and the measurement outcomes as well as the significance of the findings of this study for the various application fields. Chapter 2 Isolation and preservation methods for the epidermis and stratum corneum 16 Chapter 2 2.1 Introduction Ex vivo human skin grafts provide a cost-effective alternative to animal and clinical testing. Various companies, such as cosmetic, household product and pharmaceutical, could benefit from in vitro studies to evaluate drugs, cosmetics and other consumer products. Skin models are already used in many transdermal drug delivery and percutaneous absorption studies, as well as in irritancy and toxicology studies. Studies on ex vivo skin increase fundamental knowledge on the structural as well as mechanical properties of skin. In addition, studies on isolated skin layers, such as the epidermis or stratum corneum, could provide an insight into the specific contribution of each skin layer to the overall skin response. Skin models enable improved control of experimental conditions (i.e. temperature, hydration level) and offer the potential to perform wellcontrolled in vitro experiments. In order to obtain significant results, it is of utmost importance that the structural integrity and viability of the skin are maintained. The epidermis, the outermost skin layer, is directly contiguous to the external environment and acts as a permeable barrier. It prevents excessive water loss from the aqueous interior and protects the internal tissue against mechanical insults, UV irradiation and the ingress of foreign chemicals and micro-organisms. Due to the extraordinary nature of the epidermis, it is a challenge to completely isolate this skin layer, while maintaining its structural integrity. The keratinocytes are surrounded by a poor extracellular matrix and lack the support of a fiber structure, which usually provides the strength and elasticity in a biological tissue. Within the epidermis, the mechanical properties are determined by the rigid tonofilament cytoskeleton and the numerous desmosomes to which the filaments are anchored at the cell periphery of the keratinocytes. At the epidermal-dermal junction hemidesmosomes anchor the epidermis to the dermis (Figure 2.1). These hemidesmosomes or the adjacent anchoring filaments need to be disrupted to fully separate the epidermis from the dermis. During isolation, to maintain the complex structure of the top layer, the stratum corneum, the curvature of the skin surface needs to be followed. The architecture of the stratum corneum is widely known as a solid brick-and-mortar structure, with flat corneocytes surrounded by a matrix of lipid enriched membranes strongly held together by desmosomes. Due to the high number of plastic and cosmetic surgery procedures, such as abdominoplasty and breast reduction, the availability of ex vivo human skin is high. Whether a skin graft can be successfully used as skin model during in vitro experiments depends on the nature of the tissue. The integrity of the skin tissue mainly depends on the age of the subject as well as on the body site from which a graft is obtained. Furthermore, within one skin graft structure changes might be as a result of disease or treatments. These factors are usually reflected in tissue changes such as convolutions of the epidermal-dermal junction, thickness of epidermal strata, cell shape and surface folding, but may also lead to qualitative and quantitative differences in the various Isolation and preservation methods for the epidermis and stratum corneum 17 epidermal components [45]. To obtain the best experimental outcome from in vitro studies, it is important to use structurally and functionally intact models. In order to use the available intact skin grafts as efficient as possible, factors such as cleaning, preservation, and storage should be properly addressed, next to isolation techniques. In various studies, such as transdermal drug delivery, percutaneous absorption studies, irritancy and toxicology studies, an intact skin barrier is essential. Furthermore, proper preservation is crucial for maintaining the viability and integrity of the skin tissue. Tissue damage such as the creation of vacuoles are easily induced and the selection of a proper tissue storage method is therefore important. Evaluation techniques to assess skin viability during storage are numerous and have been extensively discussed [46,47,48]. Common methods to assess viability include Trypan blue dye exclusion, tetrazolium reductase activity, oxygen consumption rates, lactate and glucose levels, and NMR spectroscopy. Structural integrity is usually assessed by histological routines or imaging techniques. Figure 2.1: Ultrastructure of the dermal-epidermal junction. This study aims to critically review various isolation methods for the epidermis and stratum corneum and preservation methods useful for in vitro research on split-thickness skin, epidermis and stratum corneum. Some methods have already been reviewed today [49,50,51,52], but none of the reviews are up to date. No standards exist yet, complicating comparison between studies. In addition, much of the outcome of already published work may have been influenced by the used preparation technique. Studies performed in our own laboratories are added to this paper for completion. The present paper describes mechanical, ionic change, heat, enzymatic digestion and irradiation 18 Chapter 2 techniques for isolation of the skin layers. The advantages and disadvantages of each technique are discussed in terms of maintaining the skin integrity and ease of handling. In addition, the influence of various storage conditions on the skin structure and viability are discussed. 2.2 Skin preparation and analyses General steps in the preparation of skin samples used for our own experiments, are described below as well as the analysis techniques used to study the skin structure and viability. 2.2.1 Skin preparation For our own studies, human skin is obtained from female patients undergoing abdominoplasty. The research proposal for our studies was approved by the Medical Ethics Committee of the Catharina Hospital, Eindhoven, the Netherlands. Immediately after excision, the skin is brought to the laboratory for further processing. Here, the skin is placed on a stainless steel plate covered with paper towels to absorb body fluids. The skin surface is cleaned with pure water. Using multiple forceps, the skin graft is stretched and fixed to the stainless steel plate. Subsequently, split-thickness skin samples, varying in thickness from 100-400 µm, are generated using a dermatome (D42, Humeca, The Netherlands) (Figure 2.2). (a) (b) Figure 2.2: Skin is stretched using forceps (a) and dermatomed (b). 2.2.2 Histological examination In order to examine tissue structure in our laboratories, samples were fixated in 10% phosphate-buffered formalin and processed for conventional paraffin embedding. The sections were cut into 5 μm slices and stained with aldehyde-fuchsin and yellow green SF (Merckx) or standard heamotoxilyn and eosin (H&E) staining. The tissue morphology was studied by light microscopy. The aldehyde-fuchsin staining is used to clearly identify the different skin layers: stratum corneum, viable epidermis, papillar dermis and reticular dermis (Figure 2.3a). The structural integrity is examined by using the H&E staining. Isolation and preservation methods for the epidermis and stratum corneum 19 2.2.3 Analyses of skin viability Skin viability was studied by using a colorimetric MTT (Thiazolyl Blue Tetrazolium Bromide) assay. Skin samples with a diameter of 8 mm were placed in a 24 wells-plate containing 300 µl 1 mg/ml MTT solution in PBS (Phosphate Buffered Saline). The plates were incubated at 37C and 5% CO2 for a period of 3 hours. After incubation, the skin samples were removed and gently blotted with tissue paper, before completely submerging them in 2 ml 2-propanol per well. The extraction plates were placed in sealed bags to reduce evaporation and were gently shaken overnight at room temperature to extract the reduced MTT. The absorption of the extractant was measured at 570 nm using plain extractant as blank. 2.3 Epidermal isolation techniques Isolation techniques for the epidermis can be divided into the following categories: mechanical, ionic change, heat, enzymatic digestion and irradiation techniques. These techniques are discussed in this section. The success rate of the various methods are summarized at the end of the section in terms of actual cleavage plane, retaining viability and maintenance of integrity (Table 2.1). 2.3.1 Mechanical separation Cutting by using a dermatome Van Scott et al. [53] recommended a stretching method for separating the epidermis from the dermis. In this method, the skin is manually stretched to its limit over a slightly convex wooden surface, and is anchored in place by means of thumbtacks. A razor blade or scalpel is used to scrape off the epidermis. Subsequently, the epidermis is grasped by tweezers to gently detach a continuous sheet. However, damage is easily induced in the epidermis using this rough stretching technique. The severity of this damage depends on the vigour of scraping and the degree of stretching. The development of keratomes, either handheld devices or as part of a mechanical device, has improved the reproducibility of this stretching technique. In our study, we used a cordless, battery operated dermatome. As indicated in section 2.2, ex vivo skin was mounted on a stainless steel plate to facilitate the cutting process. When the dermatome was set to 100 μm, samples of the epidermis could be obtained. In some cases, however, some papillar dermis is still attached (Figure 2.3). Due to the presence of rete ridges, it is highly unlikely that the cutting plane is going through the dermal-epidermal junction only. The number of skin layers present in the separated tissue can be assessed visually; the yellowish translucent epidermis is easily distinguishable from the white opaque dermis. A MTT-test demonstrated that the dermatomed skin retained its viability, which is in agreement with Wester et al.[54]. The obtained geometric shape is very convenient for assessing its mechanical properties. It is assumed that the mechanical properties of the present papillary dermis are similar to 20 Chapter 2 the surrounding epidermal tissue, because no differences in shear properties were found between 100 and 200 μm thick split-skin samples (Chapter 3). SC SC VE VE (b) PD SC VE PD RD (a) (c) Figure 2.3. (a) Full thickness skin stained with aldehyde-fuchsin to visualize the stratum corneum (SC), viable epidermis (VE), papillar dermis (PD) and reticular dermis (RD); (b) Dermatomed skin with a set thickness of 100 μm consists of the epidermal layer only; (c) In some cases, however, some papillar dermis is still attached. Suction device Suction blisters can be produced by applying suction cups on the skin, in vivo and in vitro. In vivo separation of the human epidermis was first accomplished in 1964 [55]. Kiistala et al.(1968) found that within 130 minutes a blister with a suction gap of 25 mm can be incited. The diameter of a suction cup may vary from 15-50 mm depending on body site. To avoid tissue damage, the pressure within the cup has to be maintained at 200 mm Hg or more. The cleavage occurs in the plane through the lamina lucida, leaving the basement membrane on the dermis and retaining an intact, viable basal cell layer. However, enlargement of intercellular spaces due to considerable stretching might cause large vacuoles in keratinocytic cytoplasm [56,50]. Suction blister time depends on factors such as suction pressure, individual variation and regional differences as well as temperature, but does not depend on cup size. Because of the low reproducibility caused by individual variations that cannot be controlled, this method is considered to be unfavourable. 2.3.2 Ionic change One of the first methods to isolate the epidermis was by maceration in dilute acetic acid in order to perform mitotic counts. Cowdry [57] described that dilute acetic acid causes swelling of collagen fibers which decreases their cohesive strength and, therefore, the binding of epidermis to dermis. In addition, it was found that collagen fibers also swell Isolation and preservation methods for the epidermis and stratum corneum 21 in an alkaline environment. These methods, however, kill epidermal cells and are therefore no longer used [58]. In addition, EDTA (ethylenediamine tetraacetic acid) has been used to obtain epidermal sheets [59]. The location of the split depends on the duration of the treatment. After 30 min incubation in 0.01 M EDTA at pH 7.4 the split occurred in the lower granular layer, after 45 min it was in a spinous-suprabasilar location and after 60 min or more at the dermal–epidermal junction. Besides this, intracellular oedema is increasing with time. So this is not a favourable method for epidermal separation either. After prolonged incubation in 1 M NaCl at 4°C, the epidermis can also be easily removed from the dermis with forceps. The split occurs through the lamina lucida. Nevertheless, mitochondrial swelling was noted within the keratinocytes [50]. Although no other degenerative features have been reported, epidermal components may be diminished or modified during the long incubation times of 24 to 96 hours [60]. Prolonged incubation in PBS is also known to separate the epidermis from the dermis. After 72-96 h at 37°C, the epidermis can be readily peeled off [61]. In contrast to the above techniques, where the split occurs through the lamina lucida, the split is closer to the epidermal site of the dermal-epidermal junction [61]. Since no intact viable epidermal sheets can be obtained using techniques based on ionic change, all are considered not to be suitable for epidermal isolation. 2.3.3 Heat Separating the epidermis from the dermis using a hot plate is a simple and rapid method [58]. The skin is heated up to 50 to 60C for 30 s. To maintain enzyme activity, mild heat treatment at 52C for 30 s is required. Separation occurs at the basal cell layer. Depending on the exact conditions, release of enzymes, cytolysis and cell separation may occur. However, it has been claimed that heat does not modify fibrous proteins within isolated epidermis [62]. Heating can easily cause tissue dehydration. This problem can, however, be circumvented by increasing the humidity of the environment or by placing the skin in a sealed bag in hot water instead of using a hot plate. After heating, the epidermis can be gently peeled from the dermis. In our studies, human skin samples were heated on a hot plate and in a sealed bag. Heating the skin on a hot plate seemed to flatten the undulating epidermal structure, while the papillae remained intact after heating in a sealed bag in hot water. The epidermis could be peeled from the dermis after more than 5 minutes. For both heat separation techniques, structural tissue damage occured in terms of vacuoles and a disrupted basal layer (Figure 2.4). In addition, Wester et al.[63] demonstrated that heat treated skin (60°C for 1 minute) and heat-separated epidermis and dermis significantly lose viability. Furthermore, some practical problems arose when using a hot plate, such as curling of the dermal tissue and uneven separation of the epidermis over the complete skin surface due to gradual thermal diffusion. Lastly, it should be noted that much longer heating times were needed than mentioned in literature. 22 Chapter 2 (a) (b) Figure 2.4. Histological cross sections of epidermis isolated using heat by means of a hot plate (a) or placing the epidermis in a sealed bag in hot water (b). A standard H&E staining has been used. 2.3.4 Enzymatic digestion Trypsin Epidermal separation by means of trypsin has been widely used, although some conflicting results have been published. Briggeman et al. [64] reported that the epidermis is isolated by the cleaving effect of trypsin, whereas other authors reported that many basal cells remain loosly attached to the basement membrane after trypsin treatment [65,66]. The epidermis can be easily peeled from the dermis using 0.1-0.3% trypsin in a saline solution supplemented with calcium and magnesium at 4°C. However, these conditions also induce a high level intra-epidermal split at the spinous-granular interface [45]. Inconsistencies within the reported findings seem to be related to various factors such as size and thickness of the skin sample, enzymatic concentration and its solvent, incubation time and temperature. In addition some side-effects are not yet expressed immediately after trypsinization and post-trypsinization recovery may take up to a few days [45]. The result of epidermal isolation using trypsin depends on the specific treatment conditions in relation to the donor skin and hence, is less suitable for obtaining intact epidermal sheets. Other enzymatic methods for epidermal separation have been proven to be more consistent. Thermolysin The epidermis can easily be separated from the dermis following incubation at 4C for 1 h in a solution containing 250-500 g/ml thermolysin, a proteolytic enzyme hitherto mostly used for protein analysis [65]. Thermolysin can be dissolved in sterile magnesium free PBS containing 1 mM CaCl2 at pH 7.8. It is strongly advised to remove at least the subcutaneous fat and the lower dermis to enable the penetration of this enzyme. Light and electron microscopy revealed that the separation occurred at the lamina lucida and that the hemidesmosomes were selectively disrupted, whereas Willsteed et al.[50] noticed an intraepidermal split, without any lamina lucida separation. Since the Isolation and preservation methods for the epidermis and stratum corneum 23 introduction of this relatively new treatment, it has not been widely used. This is likely due to the gentle treatment by another enzyme called dispase. Dispase Dispase II (Roche Diagnostics) has been proven to be a rapid, effective, but gentle agent for separating intact epidermis from the dermis. This proteolytic enzyme is able to cleave the basement membrane zone region while preserving the viability of the epithelial cells [67]. Kitano and Okado were the first authors who described the seperation process [68]. Based on recommendations from the supplier, 2.4 U/ml dispase in 50 mM Hepes/KOH buffer pH 7.4 with 150 mM NaCL was used in our studies to separate the epidermis from the dermis. Fresh skin samples of various sizes were placed on top of sterile gauzes in petridishes (diameter = 6 cm) containing 5 ml of 2.4 U/ml Dispase II. The stratum corneum of the skin samples was not exposed to the enzymatic solution during the separation process to prevent loss of the skin barrier integrity. After overnight incubation at 4C and thereafter 10 min at 37C, the epidermis was gently peeled from the dermis using tweezers. It was demonstrated that the bottom surface of the separated epidermal sheet retained its rete-ridges and hair follicles with sebaceous glands and the eccrine sweat glands retained their undistorted shape [68] (Figure 2.5). The cleavage occurred in the lamina densa [69] This isolation method is very suitable for generating intact epidermal sheets. The best results were obtained when split-thickness skin samples of roughly 300 µm were used to facilitate the diffusion of this enzyme. Therefore, it is recommended to dermatome skin grafts prior to performing the enzyme treatment. Figure 2.5. H&E staining of epidermis separated with Dispase. 2.3.5 Microwave irradiation Sanchez et al. [70] explored the effects of microwave irradiation on epidermal-dermal separation. Epidermal samples were obtained after incubation in 0.02 M EDTA in PBS and microwave irradiation with 4 pulses of 420 watts for 5 sec, with a total incubation period of 4 min. The hemidesmosomal junctions are then disrupted, whereas an additional incubation time may affect keratinocyte junctions. Microwave irradiation has been widely used for tissue fixation and immunostaining. Care should be taken to avoid damage to the tissue integrity. It is important to use the prescribed buffer and specifically adhere to the recommended microwave exposure 24 Chapter 2 times. Nevertheless, microwave irradiation seems to be a rapid method for separation of the epidermis from the dermis. Table 2.1: Overview of effectiveness of isolation techniques for the epidermis. Treatment duration < 1 hr < 2hrs Suction Heat 5 min Ionic 24-96 hrs NaCl change > 1 hr EDTA 72-96 hrs PBS Enzymatic Trypsin 1-24 hr digestion 1 hr Thermolysin 24 hrs Dispase Irradiation Microwave 5 min Type Method Mechanical Dermatome Tissue Tissue Cleavage plane integrity viability Reproducibility variable + + + lamina lucida basal layer lamina lucida n.a. hemidesmosomes variable 0 0 - 0 n.a.* n.a.* 0 0 0 0 0 - hemidesmosomes lamina densa hemidesmosomes + + 0 + + n.a.* n.a.* + + *n.a. = not available 2.4 Isolation techniques for the stratum corneum Isolation techniques for the stratum corneum can be divided into the following categories: mechanical, chemical and enzymatic digestion techniques. Again the benefits and drawbacks of the various techniques are discussed. At the end of the section, the succes rate of the different techniques are summarized in Table 2.2. 2.4.1 Mechanical separation Stratum corneum separating by cutting techniques is complicated because of the skin curvature. Howevr, the thickness of the stratum corneum has little variation. So when there are means to decrease the skin curvature, mechanical separation through cutting might become possible. It was already shown that the skin relief dramatically decreases when a microscope slide is placed on top of it [71]. We performed topography measurements on unloaded and loaded skin using a PRIMOS (GFM, Germany), using light profilometry to assess the surface roughness. A piece of skin of 20x20 mm was placed on a microscope slide after removal of the subcutaneous fat layer. First, the initial surface roughness parameters were measured. Then, another microscopic glass was placed on top and pushed down by two weighs of 100 g on each side. Again the roughness parameters were determined. Preliminary testing showed that the microscopic slide on top was neglected by the system and did not influence the measurement output. A significant decrease in skin surface roughness average was measured: 42 μm in a loaded configuration versus 85 μm when unloaded. The latter is comparable to what can be found in literature [5]. Unfortunately, the surface roughness was still at least three times the thickness of the stratum corneum. Isolation and preservation methods for the epidermis and stratum corneum 25 Following the topography measurement, the sample was kept between the two plates and stored at -80°C. In order to retain the flattened state of the skin sample, the sample was cut by use of a cryotome. The surface of the stratum corneum was aligned with the cutting system to obtain stratum corneum with one single cut with a thickness of 20 μm. The stratum corneum sheets have some other epidermal strata attached and cavities (Figure 2.6). (a) (b) Figure 2.6. Stratum corneum isolated from flattened skin. Due to the skin curvature, other epidermal strata and cavities are still present. Transversal sections of the obtained sheets are depicted with 5x (a) and 40x (b) enlargement. 2.4.2 Chemical separation Cantharidin blister procedure This method, however, has only been reported up to the early seventies [22,8]. Cantharidin was impregnated into 1 cm diameter disks of filter paper and placed under occlusive patches rather than applied directly to the skin surface in a volatile solvent. The disks were removed after 4 hours and protective caps were placed over the forming blisters to prevent damage to the samples. The blister tops were surgically excised and the loose underlying wet cells removed by gentle swabbing. Since the discovery that cantharidin is toxic, it is not permitted to use it for skin treatments anymore. Ammonia vapour In the sixties and seventies, it was common to isolate stratum corneum through exposure to ammonia vapour. The latest protocols reported around 30 min exposure to separate the dermis and epidermis [72,73]. Adherent wet cells are subsequently removed with a cotton swab such that the stratum corneum sheet remains [74]. Thereafter, the stratum corneum sheet was allowed to dry on silicone-coated paper at ambient conditions. In addition, it was noticed that the success of this treatment is variable. Since more consistent techniques causing less damage became available, this method is no longer used. 26 Chapter 2 2.4.3 Enzymatic digestion Trypsin The working of trypsin throughout the epidermal strata has been extensively studied [75]. It appeared that the architecture of the stratum corneum remains unaffected by trypsinization. Corneodesmosomes and composite desmosomes shared by corneum and granular cells are normal. Tonofilaments attached to these junctions also appear unchanged [76]. However, concentrations of trypsin above 0.125% might damage the stratum corneum such that its elastic properties change [5]. In order to enable the working of trypsin on the epidermal cells, the subcutaneous fat layer and the lower dermis has to be removed. In our laboratories, the remaining skin was immersed in a porcine 0.1% trypsin (SV30037.01, Hyclone) solution in PBS (Phosphate Buffer Saline). For quick processing, the samples were then placed for over 2 hours in an incubator at 37°C. For this study, dermatomed skin of approximately 300 μm thick and a surface area of 2 cm2 was placed in 3 ml trypsin. Similar results can be obtained through an overnight culture at 4°C and 15 min at 37°C. Due to the lipids within the stratum corneum, the thin layer floats to the surface while the remaining epidermis sinks to the bottom. In order to prevent post trypsinization effects, stratum corneum is rinsed with distilled water a few times to wash out trypsin and treated with anti-trypsin. The overnight protocol can be considered as the golden standard, which is frequently described and commonly used within several research fields. (a) (b) Figure 2.7. (a) After staying overnight at 4°C, the extracellular matrix of the viable epidermis is still attached to the stratum corneum; (b) Only stratum corneum is obtained after leaving the skin sample for 1 hour at 37°C. Table 2.2: Overview of effectiveness of isolation techniques for the stratum corneum. Type Mechanical Method Cutting (cryotome) Cantharidin Ammonia Ionic change Enzymatic digestion Trypsin Treatment Tissue duration integrity Tissue viability Reproducibility 24 hrs ± - - 4.5 hrs 45 min 2-24 hrs + + ± + Isolation and preservation methods for the epidermis and stratum corneum 27 2.5 Preservation of the upper skin layers This section discusses preservation techniques regarding in vitro skin research. It is assumed that these techniques are equally suitable for all skin grafts, i.e. full-thickness, split-thickness, and epidermal grafts. From studies on skin grafts used as burn wound dressings, it is known that in order to provide the best clinical outcome, skin grafts should be properly preserved. When procuring cadaver skin for banking, the cadaver donor should be cooled as soon as possible to avoid/minimize structural tissue changes, i.e. changes in basement membrane components [77], and to maintain viability. Within 12 to 30 hours from harvest, post-mortem skin allografts exhibit an average viability index of 75% with little variation, which decreases to 40% within 60 hours. In addition, Bravo et al. [54] found that human cadaver skin grafts only exhibited approximately 60% of the metabolic activity found in fresh skin samples from living surgical donors. However, the availability of skin grafts from living donors is limited to certain body sites. Currently available methods used by skin banks for storing viable skin can be divided into short-term and long-term techniques. As a large variation in protocols have been published for storage of skin grafts and those have been extensively reviewed [77,77,78,54,79], only methods useful for in vitro testing are discussed in this section. As a consequence, some protocols that are recommended by guidelines and standards, are not taken into account when scientific studies have shown evidence that both viability and integrity are not maintained. 2.5.1 Short-term storage Due to its simplicity, cost-effectiveness and ease of availability, refrigeration of skin grafts remains the most widely used method today worldwide for short-term storage [47]. Refrigerator storage reduces the metabolic rate of the cells and hence, the nutritional demands and metabolic production. In addition, bacterial proliferation is inhibited. Without the use of preservation media, it has been reported that epidermis from porcine ear skin, which is a proper model system for human epidermis, is still in normal condition after 4 up to 6 hours at 4°C [80]. Degenerative changes started to occur at the stratum corneum and are independent of storage temperature. In contrast, the lower parts of the epidermis are generally compacted, but remained more or less structurally intact for a relatively long period. Today various isotonic media are in use for refrigerator storage (4 C) of skin grafts, which can be divided into nutrient media (e.g. HHBSS, RPMI-1640, Eagle‟s MEM with L-glutamine, McCoy's 5A) and saline solutions [75,77,78]. In general, nutrient media are considered to be a better medium than saline, as they are rich anorganic salts, amino acids, glucose and vitamins that are essential for graft viability. Mathur et al. [81] studied the preservation of viable cadaver skin grafts in PBS at 4°C. The viability was intact after 24 h of storage but rapidly declined afterwards; after 1 week the viability dropped to 27% compared to fresh skin, after 2 weeks the tissue was non-viable. In addition, the 28 Chapter 2 integrity is lost because of oedema [54]. In contrary, human cadaver skin stored at 4°C, in McCoy's 5A medium retains viability for 4 weeks [79]. Castagnoli et al. [82] demonstrated that the viability of human skin stored in RPMI-1640 media at 4°C decreased slowly, retaining 25% viability compared to that of fresh skin after 15 days of storage, with no damage to skin architecture until 7 days post-procurement. Wester et al. [63] found that the anaerobic metabolism, i.e. the conversion of glucose into lactate, of dermatomed human cadaver skin maintained a steady-state value through 8 days of culture in Eagle‟s MEM-BSS at 4°C. For percutaneous absorption studies, basal nutrient medium is preferred over growth medium containing blood serum, hormones and growth factors. The receptor fluid used within a diffusion cell, should not interfere with the analytical endpoint measurement, e.g. HPLC analyses. Recently, it was demonstrated in our laboratory that epidermis from fresh skin grafts of living donors, isolated by using a dermatome, can maintain its viability and integrity for 72 hours when maintained in HHBSS in an incubator at 37 C and 5% CO2 (data not shown). This is inagreement with results of Bravo et al .[54]. For the stratum corneum, PBS is a sufficient medium for short-term storage. In order to avoid the growth of bacteria and fungi and the loss of tissue integrity, it is recommended to store at a temperature of 4°C if it is only for a few days. 2.5.2 Long-term storage Cryopreservation Long-term storage of skin is possible via cryopreservation. In general, the success rate of freezing tissue depends on various factors, i.e. conducting medium, the cooling rate, the number of cell types in the tissue, the addition of a cryoprotective agent, storage temperature, the cooling rate and thawing rate. The viability of the epidermis (and dermis) can be well-retained when cooling to ultralow temperature by using cryoprotective agents (CPA‟s), without the formation of ice crystals. Cryporeservation is likely the most routinely used method for long-term storage of skin, because the skin can then be stored for months to years [83]. Any cell type has its optimum cooling rate producing maximum cell survival. If the cooling rate is higher than the optimum, intracellular ice appears, causing the cell to die. In contrast, if the cooling rate is slow, free water is removed from solution to form extracellular ice crystals increasing the salt concentrations in the tissue. The cells also shrink because of osmosis. It is unlikely that each cell type within a tissue will exhibit the same optimum cooling rate. Although epidermis mainly consists of keratinocytes, maintaining high viability for all epidermal cells would be challenging. This can be achieved, however, if cryoprotective chemicals are added before freezing. The most common CPA‟s are glycerol and dimethyl sulfoxide (DMSO). These cryoprotective agents act as solvents for the salts. In addition, their presence within the cells prevents excessive shrinkage of the cells during the cooling phase. Therefore in the presence of CPA‟s, it is possible to use very slow cooling rates that minimize intracellular ice Isolation and preservation methods for the epidermis and stratum corneum 29 formation while protecting the cells against solution effects. High viabilities of all cell types can be achieved using this slow cooling rate: a cooling rate of -30°C per minute was shown to maintain the viability of keratinocytes [77]. When skin tissue cryopreserved with 15% glycerol in PBS or nutrient medium has been cooled by a controlled-rate process to at least -80°C, it can be transferred for long-term storage into the vapor phase of liquid nitrogen (below -130°C). Once the skin is at a temperature lower than -130°C, i.e. the glass transition temperature of water, no further loss of cell viability is incurred. The optimum thawing procedure is a rapid warming method. This can be achieved by plunging the skin into a 37°C water bath until the tissue is just thawed. Prolonged storage at 37°C in the presence of CPA would be detrimental. Because the cells contain high concentrations of CPA, they are hyperosmotic compared with normal saline. To avoid osmotic lysis of the cells, either the saline can be added gradually or an impermeant solute such as sucrose can be added to the saline to reduce the difference in osmolarity. It has been reported that viability declines rapidly after thawing of the skin, even if the epidermis is stored in nutrient media [54]. It should be noted that it is prefered to use glycerol rather than DMSO, because it has a lower toxicity to the cells and is more effective [84,81]. Nevertheless, the skin viability might be somewhat lower after cryopreservation with glycerol [54]. Although CPA‟s are relatively non-toxic at low temperatures, the toxicity can become significant at higher temperatures. However, structurally intact skin tissue is relatively resistant to cryogenic damage compared to single cells. In addition, the rate at which CPA‟s enter the cell depends on the temperature and the CPA, being faster at higher temperatures. CPA can be best dissolved in a HEPES or TES buffer, because those zwitterionic buffers do not lose their buffering capacity at lower temperatures. Many different methods are in use for the packaging of frozen skin, ranging from rolls of skin within a tube to the use of flat pack bags in metal laminated pouches. The latter are preferred, in that the greater surface area to volume ratio ensures more even cooling across the skin tissue, and the metal laminates are good heat conductors [77]. Snap freezing Snap freezing in a well conducting medium, e.g. salt water, isopentane or hexane, provides an effectice, rapid storage method without causing structural damage due to water phase transitions. In practice, skin samples packed in a metal pouch can be emerged in a 2-methylbuthane, which is cooled down by liquid nitrogen to -80°C. The skin samples will immediately freeze and can then be stored at a -80°C freezer until use. Since this is above the glass transition temperature of water, the slow progressive decline in viability limits the maximum storage time to months. After slowly thawing at room temperature, there is no need to thaw in a buffer before using the tissue. Although the tissue is not viable anymore, the tissue integrity is well maintained. As Foutz et al. [85] showed that the mechanical properties of human skin are not affected by freezing as well, it might be sufficient to snapfreeze samples for mechanical 30 Chapter 2 characterization. Snapfrozen tissue is used for penetration and permeation studies as well. Drying stratum corneum The routinely used method of drying stratum corneum is presumably the best method to store isolated stratum corneum. According to the protocol of Bouwstra et al. [86], drying and storage should take place in a cool dark room under an atmosphere of argon or krypton. Because of possible detoriation of the lipid organization, it is recommended to adhere to a maximum storage period of approximately three months. Drying stratum corneum facilitates handling of the specimen. Most commonly is to dry the stratum corneum on filter paper, but damage may occur to the fragile sheet upon removal from the filter paper. The use of a sieve instead of the filter paper solves this problem, since the stratum corneum can be removed even dried. Before the stratum corneum sheet can be assessed, it needs to be immersed in pure water or PBS. SC epidermis Table 2.3: Overview of the ease of handle and success rate of various isolation techniques. Treatment Storage Tissue Tissue Type Method duration time integrity viability short-term saline solutions none days 0 nutrient media none weeks + + long-term cryopreservation < 1 hr years + + snap freezing 10 s 3 months + short-term PBS none 3-5 days + long-term drying few days years + + 2.6 Discussion Isolation and preservation techniques of both epidermis and stratum corneum are of importance for various in vitro studies to evaluate drugs, cosmetics and other household products. Various skin isolation and preservation techniques are commonly used today, although the effectiveness of each of these techniques has not been properly reviewed. This study provides an overview of current techniques of which the isolation methods can be divided into mechanical, ionic change, heating, enzymatic digestion and irradiation techniques for skin isolation. The study describes the advantages and disadvantages of the various methods in terms of reliability and maintaining skin integrity and viability (Error! Reference source not found. and Table 2.2). Since the cleavage plane is another indicator for the succes rate of a method, the cleavage location is also specified for each of these isolation methods. In Table 2.3, the effect of various storage conditions on the skin structure and viability are discussed. Here, the acceptable storage time is also indicated per method. Isolation and preservation methods for the epidermis and stratum corneum 31 The overview in Error! Reference source not found. shows that only few isolation methods are suitable for obtaining intact viable epidermis. Although the response of the skin to stresses such as mechanical suction and exposure to hyperosmolar salt solutions supports the concept of the lamina lucida being the natural cleavage plane of the skin [source], these methods are not recommended. The exact cleavage location due to hyperosmolar salt solutions can also be between epidermal layers, because the cleavage strongly depends on the duration of the treatment. In addition, these treatments are detrimental to the isolated epidermis. Because their protocols are also very time consuming, the techniques are incovenient for routine labaratory application as well. It was decided not to include further analysis of this method in this study to asses the effect on viability and integrity. The effectiveness of mechanical suction depends on the exact suction blister time. As the suction blister time strongly depends on various individual factors, it is considered to be impossible to obtain samples with consistent quality. Compared to the methods discussed above, isolation using heat or irradiation is much less time consuming. However, although frequently used, heat treatment does not result in an intact viable epidermis. The cleavage disrupts the basal layer, the viability declines and structural changes like cell separation have been observed. In our own lab, for gently removing the epidermis from the dermis, much longer heating times were needed than reported in the literature. This is probablyly due to specimen type or experimental conditions such as the humidity level. Due to the longer heating time, the susceptibility to structure changes and loss of viability are increased so this method is considered unfavourable as well. Isolation using microwave irradiation has been explored with satisfying results regarding tissue viability, but it is not commonly used yet. To fully assess the usefullness of microwave irradiation, more studies are needed. Three enzymes are known to induce the dermal-epidermal split. The obvious advantage of enzymatic digestion is that isolation takes place because of differences between cells meaning that the undulating structure of the epidermis and stratum corneum is followed. Trypsin, however, can cause cleavage at various planes in the epidermis making the treatment using trypsin unreliable. Thermolysin might be an alternative, but practicle studies with this enzyme are rare. What is widely used and extensively studied is enzymatic digestion using dispase. This method is very robust compared to other isolation methods: the epidermis has a consistent quality and the viability and integrity can be fully maintained. The cleavage plane is the lamina densa, so also the basal layer is completely intact. The duration of the treatment might be considered as a limitation as it is an overnight procedure. However, the number of handling steps is small and an additional advantage is that the cleavage plane is fairly independent of the treatment duration. Although it is sure that enzymatic digestion is by far the best method to isolate the epidermis, sometimes additional reasons might lead to the choice for another isolation method for certain applications. For example, the benefit of a sample with nearly perfect geometry can be more important in an in vitro set-up than experiments on epidermis only. Then, cutting slices of skin using a dermatome is an attractive quick method. Applications such as mechanical characterization benefits from the fact that the natural 32 Chapter 2 pre-stress in the skin is better retained. It was also demonstrated that the viability is retained. When looking at the options to isolate the stratum corneum from the viable epidermis, fewer methods are available from which only enzymatic digestion using trypsin gives satisfying results. It should be no surprise that both cantharadin and ammonia are harmful to the skin. Furthermore, it is without doubt that enzymatic digestion by trypsin is the common method to isolation the stratum corneum for any application field. The robustness of the method and, hence, consistent quality does not give the incentive to investigate new techniques. As it is evident that there are means to obtain intact viable sheets of epidermis or stratum corneum alone, the next challenge is to retain these properties over time. In literature, however, skin storage is mostly considered in relation to skin grafts used as burn wound dressings. As a consequence, the focus is on split-thickness skin instead of isolated skin layers, although the requirements in terms of viability and integrity are likely to be more strict for in vitro testing than for the use of burn grafts, . Preservation methods can be classified either based on technique, temperature or on storage time. The latter was chosen here because in the case of in vitro testing one can either immediately do the testing or needs to have a large batch available over a longer time. Short-term storage can usually be done in a refrigerator. Storage in an incubator at 37°C is also satisfactory. Saline solutions certainly induce tissue damage, while various nutrient media can keep the tissue viable and intact for at least a whole week before degradation slowly begins. For the stratum corneum, it makes sense to use a saline buffer. However, it is advised to do this only when using the samples within a week. For longer storage, stratum corneum should be dried under the right conditions, as infections and fungi may easily grow. In the long-term, cryopreservation is a routine laboratory technique which can produce large batches which can be beneficial for years. There is a risk of inconsistent quality of the samples due to the sensitivity for tissue damage during thawing. When viability is not requirement, snap freezing is a convenient and reliable method for long-term storage. Although the variety of topics for in vitro skin research is enormous, this review has shown that the isolation and storage protocols can be identical. Future in vitro research should make use of isolated epidermis, which is separated by the enzyme dispase or cut using a dermatome because of its convenient geometry. When the epidermal samples are subsequently stored for just a short period and tissue growth is not the goal, it is advised to use a nutrient media such as HHBSS. For long-term storage, the only option for intact viable tissue is cryopreservation. Regarding the stratum corneum, trypsin and drying remain by far the best methods to isolate and preserve this skin layer. Isolation and preservation methods for the epidermis and stratum corneum 33 Acknowledgements First of all, we would like to thank professor Bouwstra for her contribution in the discussions. We are also very grateful to professor Hagisawa for providing the aldehydefuchsin staining procedure. Last, we would like to thank the plastic surgery department of the Catharina hospital in Eindhoven for providing the skin tissue. Chapter 3 Linear shear response of the upper skin layers 36 Chapter 3 3.1 Introduction Knowledge about the mechanical behavior of human skin is of great importance for various clinical and cosmetic treatments. The human skin is composed of a non-uniform layered structure and the mechanical behavior of all the layers is highly complex: i.e. anisotropic, inhomogenous, non-linear and viscoelastic. Therefore, the most appropriate approach seems to be to determine the mechanical properties of each individual skin layer all loading directions in order to understand the full skin response. The present study focuses on the contribution of the outer skin layer, the epidermis, when in-plane forces are applied to the skin surface. Because of the anisotropic nature of the epidermis, the response in tensile and shear are most probably very different. Usually, tensile properties are addressed in research studies. However, the shear component plays a key role in applications such as the development of pressure sores, the removal of skin adhesives and skin-device contact such as with prosthetic limbs and shavers. The collective shortcoming for all these applications is the poor knowledge of the mechanical response of the epidermis to shear, obstructing the further improvement of current treatments and devices. As the epidermis is the chemical and physical barrier between the human body and its environment, it possesses extraordinary structural properties. Epidermis is a stratified epithelium, consisting of four different layers, defined by position, shape, morphology and state of differentiation of the keratinocyte, the main cell type. The epidermal tissue is renewed constantly: cells are lost from the skin surface by desquamation and this loss is balanced by cell division and growth in the basal layer [87]. The most superficial layer, the stratum corneum, has a thickness of typically 10-20 μm, and is considered as a separate layer because of its specific barrier function. The stratum corneum has a „brickand-mortar‟ structure with the corneocytes, which are differentiated non-viable keratinocytes, as „bricks‟ in a „mortar‟ of lipid membranes and desmosomes. The thickness of the remaining part of the epidermis, the viable epidermis, ranges from 30100 μm. To strengthen the attachment of the epidermis to the dermis, the junction has an undulating shape resulting in large cones of epidermal tissue penetrating the dermis. The properties of both viable epidermis and stratum corneum are influenced by environmental conditions such as temperature (T) and relative humidity (RH). Usually, load-bearing soft tissues are composed of a fiber network, providing the strength and elasticity to the tissue, but this is not the case for the epidermis. Its extensibility is mainly due to the possibility to smooth out the skin surface, while the strength and cohesiveness are due to the rigid tonofilament cytoskeleton and the numerous desmosomes at the periphery of the keratinocytes. Furthermore, the viable epidermis is a very compact tissue; the intercellular spaces occupy less than 2% of the volume [5,13]. Consequently, the viable epidermis is suspected to be more rigid than other soft tissues. In the stratum corneum, the cellular membranes are thickened, the Linear shear response of the upper skin layers37 water content is decreased and a larger amount of keratin is present and thus, its mechanical stiffness and strength are suggested to be even higher. Due to the complex skin structure, the mechanical response of the epidermis cannot be easily distinguished from that of the dermis in an in vivo experiment. This results into two important implications for mechanical characterization of epidermis: 1) skin layers need to be measured individually, and 2) in vitro measurements are required. Regarding the first implication, stratum corneum and the entire epidermis can be isolated from other skin layers, but there are no means to isolate viable epidermis. So both isolated and combined skin layers need to be characterized to assess the mechanical response of the viable epidermis. Furthermore, in vitro measurements opens up a broad range of reliable standard techniques used in mechanical engineering. Nevertheless, these methods need to be adapted to enable the measurement of thin layers of soft materials. Moreover, issues regarding the complex sample geometry, the heterogeneous tissue composition and the sensitivity to environmental conditions have to be dealt with. Currently, there is a paucity of papers describing mechanical properties of the entire epidermis or viable epidermis only. Studies so far were either on a small-sized scale [37,88], not reproducible [89] or included the total papillar dermis [38] and none of them investigated the shear response. Mechanical properties of stratum corneum have been studied and reviewed more extensively [34,90,5,21]. However, also for the stratum corneum, very few studies are investigating shear properties. Consequently, quantitative shear data for the upper skin layers is sparse or not existent. It is hypothesized that the shear modulus of the epidermal layers is far below the broad range of tensile Young‟s moduli found in literature because of the anisotropic structure of epidermis. We measured the mechanical behavior of various human skin layers subjected to shear over a wide frequency range and with varying environmental conditions, i.e. temperature and relative humidity (RH). Because of the complexity, we limit ourselves in this study to determine the small strain behavior of stratum corneum and viable epidermis. To validate the experimental approach, also tests with silicone rubbers are performed. 3.2 Methods 3.2.1 Sample preparation Skin Skin was obtained from patients undergoing abdominoplastic surgery, who gave informed consent for use of their skin for research purposes under a protocol approved by the ethics committee of the Catharina Hospital, Eindhoven, The Netherlands. Only abdominal skin of Caucasian women from a age group between 35 and 55 years old is used. Abdominal skin with stria, cellulite, damage due to UV exposure or tremendously hairy skin is excluded from the study. 38 Chapter 3 Immediately after excision, the skin is brought into the laboratory and processed within 4 hr. Skin slices are obtained using an electric dermatome (D42, Humeca, The Netherlands) of which the set thickness was refined for this purpose by the supplier. In order to separate the epidermis, the thickness is set to 100 μm. Subsequently, circular tissue samples of the epidermis are obtained from the slices using an 8 mm diameter cork borer. The epidermis is estimated to vary from 50 to 150 μm on this body site [12,5]. Depending on various factors such as skin surface roughness, tissue hydration, smoothness of the cutting, some papillar dermis could remain attached (Figure 3.1). To obtain stratum corneum, dermatomed skin slices of 300 μm are also punched into 8 mm diameter samples before immersion in a solution of 0.1% trypsin (SV30037.01, Hyclone) in PBS at 37°C for 2-3 hr. Thereafter, samples are rinsed with PBS. Also split-thickness skin of 200 and 400 μm in thickness is obtained using the dermatome. As can be seen in Figure 3.1, the 200 μm split-thickness skin is composed of epidermis and papillar dermis. In the 400 μm split-thickness, also reticular dermis is included. For isolating the reticular dermis, the top layer of skin is dermatomed until the white opaque dermis is on top. Then, a 400 μm thick layer of reticular dermis is dermatomed. The stratum corneum samples were stored in PBS at 4°C for maximal 7 days but dried when longer storage is needed. All other samples were stored in a Hank‟s Hepes Balanced Salt Solution (HHBSS) for a maximum of 72 hrs in an incubator until use. The viability of the samples was determined by a standard colometric MTT (Thiazolyl Blue Tetrazolium Bromide) assay. The tests proved that the tissue viability does not change after a storage period of 72 hours (data not shown). Silicone rubber In order to validate the experimental approach, a highly elastic silicone rubber (Köraform 42 A , Alpina Siliconee, Germany) was chosen. The silicone rubber was poured under vacuum into various thicknesses: 0.05, 0.12 and 2.00 mm. Circular samples were obtained by using an 8 mm diameter cork borer. 3.2.2 Experimental set-up All experiments are performed on a rotational rheometer (ARES, Rheometric Scientific, USA) with parallel plate geometry in combination with a Peltier environmental control unit and a fluid bath. Plates are sand-blasted to prevent slippage. An eccentric configuration is used, where the sample is placed at the edge of the plate with a radius of 33 mm (Figure 3.2), allowing for the measurement of soft tissues [91,92,93]. The shear stress 𝜏 and shear strain 𝛾 are then calculated from the measured torque 𝑀 and the angle 𝜃 by: 𝜏 = 2𝜋𝑟12 𝑀𝑟 𝑟 − 𝑟1 2 2 + 𝑟12 8 , 𝑟 𝛾=𝜃 , ℎ (3.1) Linear shear response of the upper skin layers39 SC VE PD (b) (a) RD (c) (d) Figure 3.1: Histological cross-sections of dermatomed skin: (a) 100 μm split-skin with stratum corneum (SC) and viable epidermis (VE), (b) 100 μm split-skin containing epidermis and some papillar dermis (PD), (c) 200 μm split-skin consisting of epidermis and papillar dermis, (d) 400 μm split-skin including reticular dermis (RD). M r1 r Figure 3.2. Eccentric configuration for rotational shear experiments. A sample with radius 𝒓𝟏 is rotated at a radius 𝒓 with a torque 𝑴. The groove following the perimeter facilitated the positioning of the samples. 40 Chapter 3 where 𝑟 is the radius of the plate, 𝑟1 is the sample radius and ℎ is the sample height. The advantages of shifting the sample to the edge of the plate are that the measured torque signal is increased and the deformation is more homogeneous than in the conventional centered configuration. Samples are gently placed in the correct position by using tweezers. In order to spread out the stratum corneum sample, a droplet of PBS is placed in which the stratum corneum sample unfolds. Subsequently, the droplet is extracted by using a tissue. The other skin samples can be placed using tweezers only. Visible droplets on the surface of all sample types are gently removed. Next, the upper plate is lowered until the sample experienced the intended normal force. Samples are measured in a controlled environment using a home-built system, see Figure 3.3. Via a pressure switch, dry compressed air enters two channels, of which one channel conducts through a chamber filled with water to obtain fully humidified air. In the next chamber, dry and fully hydrated air are mixed to obtain the desired RH by regulating the flow inlets. The mixing chambers are placed in a water bath to control the temperature. Finally, the air is brought via a temperature controlled tube (HT 20, Horst GmbH, Germany) into the measurement chamber, in which the temperature is controlled through the air inlet as well as via the bottom plate by the Peltier environmental control unit. A RH/T-sensor (Hytemod-USB, Hygrosense Instruments GmbH, Germany) is located near the sample. G F E A D C B Figure 3.3: Measurement set-up. Pressurized air goes via the pressure switch (A), whereafter the air is split up into two tubes, passes flow regulators (B) and flow meters (C), before entering the humid and/or mixing chambers in the waterbath (D). Then, the air goes via a temperature-controlled tube (E) into the measurement chamber of the rheometer (F), where a RH/T-sensor (G) is giving feedback about the actual RH and temperature. Linear shear response of the upper skin layers41 3.2.3 Rheological methods Linear viscoelastic material behavior is described by a multi-mode Maxwell model: 𝜏𝑖 + 1 𝜏 𝜆𝑖 𝑖 = 𝐺𝑖 𝛾 ; 𝑖 𝜖 1, 𝑛 (3.2) where 𝜏𝑖 is the shear stress contribution of mode 𝑖 with the relaxation time 𝜆𝑖 and modulus 𝐺𝑖 . The applied strain rate is denoted with 𝛾 . The total stress (𝜏) is the sum of the stress contributions of all modes: 𝑛 𝜏= 𝜏𝑖 (3.3) 𝑖=1 A frequency (𝜔) dependent input 𝛾 = 𝛾0 sin 𝜔𝑡 will lead, for linear viscoelastic behavior, to a sinusoidal shear stress: 𝜏 = 𝐺𝑑 𝛾0 sin(𝜔𝑡 + 𝛿), (3.4) where 𝐺𝑑 (𝜔, 𝑇) is the dynamic modulus and 𝛿(𝜔, 𝑇) the phase shift. The response can be written in an in-phase and out-of-phase wave: 𝜏 = 𝜏 ′ + 𝜏′′ = 𝜏0′ sin 𝜔𝑡 + 𝜏0′′ cos 𝜔 𝑡 (3.5) From this, the moduli can be computed: 𝑛 ′ 𝐺 = 𝜏0′ 𝛾0 = 𝑖=1 𝑛 𝜆2𝑖 𝜔2 𝐺𝑖 1 + 𝜆2𝑖 𝜔 2 𝐺 ′′ = 𝜏0′′ 𝛾0 = 𝐺𝑖 𝑖=1 𝜆𝑖 𝜔 1 + 𝜆2𝑖 𝜔 2 (3.6) (3.7) where 𝐺 ′ is the storage modulus, representing the elastic part of the behavior and 𝐺 ′′ is the loss modulus, representing the viscous behavior. The two moduli, 𝐺 ′ and 𝐺 ′′ , form the dynamic shear modulus: 𝐺𝑑 = 𝐺′2 +𝐺′′2 The phase shift 𝛿 to 𝐺′ and 𝐺" via; (3.8) 42 Chapter 3 𝐺 ′′ tan 𝛿 = 𝐺′ (3.9) 3.2.4 Experimental procedures The ultimate goal of this study is to determine the loss and storage moduli of stratum corneum and viable epidermis as a function of frequency, temperature and relative humidity (RH). If skin layers can be isolated, they are measured separately. If not, measurements are performed on combinations of skin layers. In order to determine the mechanical parameters, the linear viscoelastic strain regime, i.e. the strain range in which the material properties are independent of the strain amplitude, has to be identified. Moreover, the typical characteristics of the upper skin layers makes that other preliminary tests are essential, i.e. the right experimental conditions needs to be defined to ensure reliable results. First of all, the samples, especially the stratum corneum samples, are extremely thin (under 20 μm). Measuring such thin samples is at the limit of the possibilities of the apparatus used. Therefore, to validate the experimental approach, a well-defined homogeneous soft material, i.e. silicone rubber, with different thicknesses was tested. Also, the approach of using a stack of layers to increase the sample thickness was tested. Furthermore, the natural wrinkling shape of the thin sample (see Figure 3.1) may cause contact problems between the sample and the parallel plates. Flattening the wrinkles may reduce these contact problems. Therefore, the influence of the level of normal force applied was determined for various numbers of stratum corneum samples stacked. Last but not least, the sensitivity of the upper skin layers to its environment needs to be translated into conditioning times, i.e. the times required for stationairy mechanical behavior. The experimental procedures for the topics mentioned are discussed in the order given below. For each experimental procedure is stated which types of samples are used. validation of the experimental approach (silicone rubber) stacking (stratum corneum) determination of the linear viscoelastic strain regime (stratum corneum, epidermis, epidermis + papillar dermis, epidermis + dermis, reticular dermis) determination of the conditioning time (stratum corneum, epidermis) determination of linear viscoelastic properties over a frequency range as a function of temperature and humidity (stratum corneum, epidermis). Validation of the experimental approach In order to validate whether the experimental method applies for thin samples using this measurement set-up, experiments are conducted on silicone rubber samples with varying thickness but similar diameter (8 mm). The shear modulus is determined for various frequencies increasing stepwise from 1 to 100 rad/s at 0.01 strain. Linear shear response of the upper skin layers43 Stacking A possible way to resolve the problem of thin samples with a complex wrinkled sample geometry is to stack a few of these on top of each other. This approach is checked for 1,3 and 5 layers of dried stratum corneum, respectively. First, the dried samples are conditioned at room temperature for 1 hr. The normal force is varied between 1-10 g, measuring the corresponding thickness and the shear modulus at 10 rad/s and 0.01 strain at the same time. The measurements are performed at room conditions (50% RH, 22°C). As the other skin layers are thicker and more pliabble than stratum corneum, it is assumed that the space between the plates is better filled up and that the skin surface roughness is negligble. A normal force of 1 g is applied on these samples. Linear viscoelastic strain regime The linear viscoelastic strain regime can be determined using oscillatory shear experiments with constant frequency and varying strain (strain sweep). The strain sweeps are performed at 10 rad/s for strains varying from 0.001 up to 0.1 at room conditions (50% RH, 22°C) on all skin sample types: e.g. stratum corneum, epidermis, epidermis and papillair dermis, epidermis and dermis and reticular dermis only. As the samples are already placed in the room for over 1 hr, it is assumed for now that 20 minutes conditioning in the closed chamber of the measurement set-up prior to the start is sufficient. Samples consisting of only reticular dermis are measured in a humid environment to prevent dehydration. Conditioning times Conditioning times are derived from oscillatory shear experiments with a strain of 0.01 at 10 rad/s for 1 hr at various RH at 22°C. These time sweep series are performed on stratum corneum and epidermis. Data points are collected every 30 s. Determination of linear viscoelastic properties The previous tests should prove that the experimental approach enables the measurement of the small strain behavior of epidermis and stratum corneum. As a result, frequency sweeps ranging from 0.1-100 rad/s at 0.01 strain can be applied at 25%, 50%, 75% and 98% RH and 22°C and 37°C. Conditioning time varies from 20 minutes at 25% RH, 35 min at 50% RH and 75%RH, up to 45 min for 98% RH. 3.2.5 Histological examination Histological examination of the used samples provides a control for the thickness measurements with the rheometer, the skin layer composition and for abnormalities. All samples are fixated in 10% phosphate-buffered formalin after mechanical testing and processed for an aldehyde-fuchsin staining. The sections are cut at 5 μm and stained with aldehyde (Merck) and light-green (Merck). The tissue morphology and sample thickness is studied by light microscopy. 44 Chapter 3 3.3 Results For all tests, the linear viscoelastic behavior is presented in terms of the shear modulus, 𝐺𝑑 , and the phase angle, 𝛿. Because it appeared that 𝛿 remains constant for all measured conditions, these data are not always displayed. Validation of experimental approach In order to prove that the experimental approach is appropriate for thin samples, frequency sweeps were applied for silicone rubbers of varying thickness. The results are shown in Figure 3.4. No significant differences between the storage modulus 𝐺 ′ and the loss modulus 𝐺" for the various thicknesses are measured. When assuming NeoHookean material, such that 𝐸 = 3𝐺𝑑 , the derived Young‟s modulus is also similar to those obtained from tensile testing. It is conluded that the experimental approach is appropriate for measuring thin, soft materials. Figure 3.4: Frequency sweeps performed on silicone rubber of various thickness: 0.050, 0.120 and 2.00 mm. Stacking In this test, stratum corneum samples were studied. As shown in Figure 3.5a, increasing the force from 1 to 3 g results in large differences for the measured gap in the measurement set-up, indicating that the wrinkling surface is unfolded. Increasing the force from 3 g up to 10 g causes relatively small deformations, indicating compression. Thus, a normal force of 3 g applied on one stratum corneum sample of 8 mm in diameter should provide sufficient contact between the sample and the parallel plates. The constant value of the shear modulus at this normal force in relation to the number of stacked samples supports this conclusion (see Figure 3.5b). Linear viscoelastic strain regime As shown in Figure 3.6, the linear viscoelastic strain regime is similar for stratum corneum, epidermis, dermis and split-thickness skin. For all those skin types, it is observed that the shear response is independent of the applied shear strain until nearly 0.01. As the conditioning time for epidermis and stratum corneum could only be estimated during this test, the measured value of 𝐺𝑑 might slightly differ from the actual 𝐺𝑑 when those skin layers are involved. Therefore, data shown are normalized. It should be noted that the value of 𝐺𝑑 for the reticular dermis is much less than for skin samples including epidermis. Furthermore, the measured gap could deviate more than 50% from the set thickness of the dermatome for samples containing epidermis and Linear shear response of the upper skin layers45 dermis (not shown). However, histological examination showed that the composition of those skin samples is in agreement with the expectations. (a) (b) Figure 3.5: The effect of stacking dried stratum corneum samples: (a) total sample thickness vs. the measured gap at various normal forces: the dotted line represents the linear relationship between gap and number of stratum corneum (SC) samples stacked; (b) the shear modulus at varying axial forces vs the number of SC layers at a frequency of 10 rad/s: the dashed line represents the average of the measurements using an normal force of 3 g (). Figure 3.6: The normalized 𝑮𝒅 of the average results of strain sweeps performed on various skin layers. For each skin layer, 3 samples from each of the 3 specimens were tested. Conditioning To reduce measurement time, the conditioning times were identified for epidermis. Since the thicker epidermis needs more time to adjust to a certain temperature and humidity, it is assumed that its conditioning time also holds for stratum corneum. The results of the time sweeps are depicted in Figure 3.7. At low RH, the mechanical response is stabilized within 20 minutes. Since hardly any difference is observed between the settling times for 50% and 75% RH, both conditioning times are set at 30 minutes. At 98% RH, the moduli 46 Chapter 3 slightly decrease until about 40 min. Therefore, fully hydrated skin samples are preferably conditioned for 45 minutes. A considerably increase in the standard deviaton for the higher RH was noted. Figure 3.7: Average values of 3 measurements for 𝑮𝒅 (𝝎 = 𝟏𝟎𝒓𝒂𝒅/𝒔, 𝑻 = 𝟐𝟎°𝑪) and the standard deviation over time (dotted lines) for the epidermis at various RH. The vertical grey band indicate the necessary conditioning time. Determination of G’ and G” The dependency on RH and temperature were measured for both the epidermis and stratum corneum (Figure 3.8 and 3.9). For each RH/T combination, it was aimed to measure 3 samples per subject. However, the test sequence could not be completed for subject 2 and 4 within 72 hr. Subject 4 was therefore excluded from the study on epidermis. For both stratum corneum and epidermis, the modulus is slightly frequency dependent (see Figure 3.8). The phase angle is not significantly different for the various RH. As similar results were obtained for epidermis and stratum corneum, only results from the latter are shown in Figure 3.8. Because of this mild frequency dependency, a comparison between the different environmental conditions could be done at one frequency only. In this case, we chose 10 rad/s (see Figure 3.9 and Figure 3.10). The results for stratum corneum show a decrease in modulus with increasing humidity, but no correlation with temperature. For the epidermis, data were less consistent, especially at 20°C. It is suggested that its mechanical properties are independent for small temperature changes. Linear shear response of the upper skin layers47 (a) (b) Figure 3.8: Linear viscoelastic behavior of stratum corneum from one subject for various RH at 20°C. (a) The average shear modulus 𝑮𝒅 ; (b) The average phase angle δ. Figure 3.9: Linear viscoelastic behavior of the stratum corneum for various RH at 20°C and 37°C. The average values and standard deviations are shown for 𝑮𝒅 and δ per subject. 48 Chapter 3 Figure 3.10: Linear viscoelastic behavior of the epidermis for various RH at 20°C and 37°C. The average values and standard deviations are shown for 𝑮𝒅 and δ per subject. 3.4 Discussion In the past, mechanical behavior of epidermis has been described qualitatively due to the lack of experimental data. The skin curvature and the undulating dermal-epidermal junction cause inherent difficulties during mechanical characterization of the epidermis in vivo. In addition, in vivo measurement methods for shear, such as elastography, cannot be applied (yet) due to limitations in resolution. Therefore, this study presents an in vitro measurement method to determine shear properties of the epidermis. Preliminary testing was essential to validate the methods applied and to obtain the right experimental conditions allowing for reliable final measurements. In order to measure shear properties of a soft biological material, measurement methods as developed for muscle, brain and thrombus [91,92,93] could be used. However, pretesting was needed to prove that the experimental approach is also appropriate for samples within the order of a few micrometer, while retaining a relative large diameter to avoid the effect of local properties. As there are inherent difficulties in determining the actual thickness of stratum corneum from histology, the sample thickness was defined by the measured gap between the plates. Although the thickness of the stratum corneum on the abdomen is reported to be 14±4 μm [5], the measured gap at a normal force of 3g varied from 15 to 60 μm due to local variations and skin surface roughness. Applying a Linear shear response of the upper skin layers49 higher normal force causes compression of the sample, which influences the measured modulus, and, therefore, should be avoided. The skin surface roughness becomes less significant for the other thicker skin layers. In addition, these layers are more pliable than stratum corneum. Whether stratum corneum, epidermis only or epidermis and dermis together are measured, the shear response does not differ significantly for the strain sweeps. It is hypothesized that loading in shear causes cell deformation in the epidermis while hardly affecting the desmosomes. As inplane shear hardly effects the transversal dermal fibers as well and few cells are present in the dermis, the dermal response will be mainly due to ground substance deformation. It is likely that this substance has a lower shear resistance than the highly organized epidermis. In contrast, the tissue response on in-plane tensile loading will be determined by the strength of the desmosomes, the elasticity of the dermal fibers and the direction of the Langer lines. Recently, the linear viscoelastic response on oscillatory shear strains of human whole skin and dermis-only was measured [94,95]. The increase of the moduli was more pronounced for the dermis-only at higher frequencies, so the authors concluded that the epidermis is only slightly frequency dependent. At lower frequencies, 𝐺𝑑,𝑑𝑒𝑟𝑚𝑖𝑠 was in the order of kPa. In accordance to this study, we observed in our frequency sweeps that the epidermis is indeed slightly frequency-dependent. Our strain sweeps also resulted in a value for 𝐺𝑑 ,𝑑𝑒𝑟𝑚𝑖𝑠 of a few kPa. For the stratum corneum, the values of 𝐺𝑑 are similar to these of the epidermis, but might be somewhat differented when corrected for uncertainties in the sample thickness. The results for epidermis and stratum corneum suggest that the small strain shear properties of viable epidermis and stratum corneum hardly differ. Currently, our shear moduli can only be compared with in-plane tensile properties of stratum corneum from literature. When doing so, our shear moduli are one order of magnitude below the properties in dry conditions and up to two orders of magnitude when fully hydrated when using the lowest reported values of the Young‟s moduli [22,24,96]. This clearly supports the highly anisotropic behavior of stratum corneum and epidermis. A decrease in stiffness of the stratum corneum could be observed with increasing RH. In accordance with our observations, delamination studies with stratum corneum, which also showed the pre-failure mechanical response, showed no temperature-dependence for this temperature range [97]. No clear relationship between the mechanical properties of the epidermis and RH could be established. Time sweeps showed that moduli set after a certain conditioning time. However, both time sweeps and frequency tests for epidermis showed larger variations per RH and per subject compared to stratum corneum. This might be related to the less well-defined tissue composition. For example, the direction of Langer lines or irregularities such as sweat pores and hair follicles can have a more substantial role in the mechanical behavior in fully hydrated epidermis than for stratum corneum. Future experiments should clarify the variance in these results. Longer conditioning times and larger variations were observed in fully hydrated epidermal samples than for less humid samples. Examination of fully hydrated SC 50 Chapter 3 structure has revealed swollen corneocytes and water pools in the extracellular spaces after storage in PBS [98]. Furthermore, water disrupts the lipid lamellae to varying degrees and causes degradation of intercellular corneosomes [87,87,99]. It is likely that the desmosomes in the viable epidermis are also highly susceptible to damage. However, histological examination did not show any sign of degradation in our samples. The prolonged time of conditioning the sample in the set-up at higher RH limited the number of experiments that could be done with epidermis from one donor within 72 hours. The present study demonstrated that reproducible results can be obtained for the shear properties of epidermis in an in vitro set up. Viable epidermis could not be measured as an isolated skin layer, but its properties can be derived from the other skin samples tested. The 𝐺𝑑 for stratum corneum roughly ranges from 4 to 12 kPa, decreasing with increasing RH. The values are far below the shear modulus value based on tensile Young‟s moduli (i.e. 𝐸 = 𝐺𝑑 ) in literature, assuming anisotropic material behavior. Results for the epidermis were in the same order of magnitude, but was less consistent. A reason might be the less well-defined tissue composition. Therefore, it would be interesting to combine mechanical testing with real-time imaging techniques to follow changes in tissue deformation. It was already shown by histological examination after 2 days of loading that shear forces induce cell displacement in skin, and particularly in the epidermis [100]. Furthermore, electron microscope imaging techniques could support histological examination in assessing tissue damage due to preparation, storage or handling. Last but not least, the shear response needs not only to be correlated with the tensile loading but also with the effects of perpendicular loading, such as indentation or compression. Chapter 4 A new indentation method to determine the mechanical properties of epidermis 52 Chapter 4 4.1 Introduction The outer skin layer possesses important characteristics that make them a favorable site for pain-free drug delivery with minimal damage: a rich population of immunologically sensitive cells as well as the lack of blood vessels and sensory nerve endings. Today, the development of drug delivery using microneedles or microjets is challenging. because of the poor understanding of the mechanical behavior of the human skin layers. In particular, the key mechanical properties of the outer skin layer, i.e. the epidermis composed of stratum corneum and viable epidermis, should be better understood. The structure and function of this layer are well-known [10]. The outer layer, the stratum corneum. is an effective physical barrier of dead cells in a „brick-and-mortar‟ structure: the anucleate corneocytes form „bricks‟ and the intercellular lipid membranes and corneosomes are considered as mortar. The viable epidermis mainly consists of migrating keratinocytes towards the stratum corneum, continuously changing in composition, shape, and function. The junction with the underlying dermis is strengthened by its undulating pattern such that large cones of epidermal tissue penetrate the dermis (see Figure 4.1). Furthermore, epidermal properties are influenced by environmental factors such as temperature, humidity and UV radiation. In order to deliver drugs transdermally, the microneedle or nozzle should penetrate the stratum corneum to deliver the drug 100-150 μm below the skin surface, e.g. in the viable epidermis or papillar dermis. It is therefore important, besides penetration studies, to investigate the mechanical behavior of epidermis to understand the delivery path through the epidermis as well as the tissue repair and remodeling mechanisms associated with the treatment. Until today, however, studying mechanical properties of skin was limited to dermis and stratum corneum, ignoring viable epidermis. As sharp indentation leads to the penetration of a microneedle or nozzle, the specific interest in this study are the mechanical properties of the epidermis during indentation at a micron lengthscale. Recently, Kendall et al. [37] were the first publishing mechanical properties of the (viable) epidermis during penetration, using modified standard tips on murine skin. They observed a decrease in storage modulus when the 2 μm probe penetrates through the stratum corneum, which is in accordance with studies on stratum corneum only [101,102]. The authors argued that this is because of an increasing moisture content with depth. In the viable epidermis, the storage modulus remained nearly constant. In contrast, penetration of the 5 μm probe showed a negligble decrease in storage modulus throughout the stratum corneum and a gradual increase in the viable epidermis but still below the shear modulus values for the 2 μm probe. A variety of in vivo and in vitro indentation techniques were developed to measure the stratum corneum. In the eighties, Hendley et al. developed an indentation device to measure force variations in vivo due to age, sex and body site [102]. A needle of 11 μm at the tip was held perpendicular to the surface and moved rapidly into the skin. They claimed that the speed of the indentation ensures that predominantly stratum corneum properties are tested [33]. Measured forces were typically in the order of 3.0 N. Recently, A new indentation method to determine the mechanical properties of epidermis 53 some nano-indentation studies have been performed on isolated stratum corneum [103,31,104,104,101]. The tips used varied between 1-10 μm, while corneocytes have a diameter ranging from 26-45 μm [105,3,106]. As a consequence, very local properties are determined in experiments using those tips. Furthermore, in some of the studies, the three-sided Berkovich tip that come to a sharp point is used. This tip easily induce damage on the sample‟s surface, which interferes with the load-displacements results of the indentation. Three of the nano-indentation studies were based on continuous stiffness measurements (CSM) protocols [37,101,104]. The drawback of CSM is that the results are influenced by the selected amplitude and frequency for viscous materials. Taken all nano-indentation studies on stratum corneum together, the measured Young‟s moduli varied from 10 MPa [107] for wet porcine samples up to 1 GPa for dried human samples [101]. This broad range is likely caused by the differences in testing apparatus and protocols, differences between species and body sites, and the heterogeneity of the material. A reliable method to determine the mechanical properties of the stratum corneum only on the tissue level is therefore also required. The objective of the present study is to determine Young‟s modulus of the epidermis, e.g. the stratum corneum and viable epidermis, by means of a micro-indentation test. The typical complex geometry, undulating and less than 150 μm in thickness, and the porosity of the epidermis put high demands on the mechanical characterization. Therefore, isolated epidermis and isolated stratum corneum were tested using equipment that is known for its accuracy and reliablity. As the used device was originally designed for solid materials of which well-defined samples can be obtained, the protocol was adapted to be applicable. To validate that the testing protocol holds for thin materials with a low stiffness, tests have been performed with silicone rubber. 4.1.1 Sample preparation Skin Indentation tests have been carried out on ex vivo abdominal skin of Caucasian women from a similar age group (43±4 years old) undergoing abdominoplasty surgery. All patients gave informed consent for use of their skin for research purposes under a protocol approved by the ethics committee of the Catharina Hospital, Eindhoven, The Netherlands. Abdominal skin with striae markers, cellulite, damage due to UV exposure or excessively hairy skin is excluded from the study. Immediately after excision, the skin is brought into the laboratory and processed within 4 hours. Epidermal sheets were obtained using a dermatome (D42, Humeca) of which the set thickness was refined for this purpose by the supplier. The dermatomed slices of 100 μm thickness were cut in pieces of approximately 1 cm2. Depending on various factors such as skin surface roughness, tissue hydration, and the amount of cones and ridges, samples may consist of epidermis and/or some papillar dermis [Figure 4.1]. To obtain stratum corneum samples, dermatomed skin slices of 200 μm were immersed in a solution of 0.1% trypsin (Hyclone, SV30037.01) in an incubator at 37°C for 2-3 hr. 54 Chapter 4 Thereafter, the sheets were rinsed in PBS and also cut into pieces of approximately 1 cm2. All samples were stored at -80°C until further use. SC SC VE VE (b) PD SC RD VE PD (a) (c) Figure 4.1. An aldehyde-fuchsin staining is used to visualize the morphology of the various skin layers: (a) Full-thickness skin including the stratum corneum (SC), viable epidermis (VE), papillar dermis (PD) and reticular dermis (RD); (b) Dermatomed skin with a set thickness of 100 μm consisting of the epidermal layer only; (c) Dermatomed skin of 100 μm consisting of epidermis and some fragments of papillar dermis. Silicone rubber In order to validate that the experimental procedure is valid for thin samples, a highly elastic silicone rubber (Köraform 42 A, Alpina Siliconee, Germany) was measured using various sample thicknesses. The silicone rubber was poured under vacuum into various thicknesses: 0.05, 0.12 and 2.0 mm. Then, samples of about 1 cm2 were cut out. 4.1.2 Experimental procedure Skin The skin sample was placed on a substrate such that in-plane tissue movement cannot occur. The large number of pores in the epidermis hardly allowed any fixation method. It appeared, however, that the adhesive, sticky behavior of the skin sample is strong enough so no other fixation was required. Immediately after thawing at room temperature, samples were spread out on an aluminum disc with the outer skin surface facing up. Possible air or liquid below the tissue was gently squeezed out. The samples were allowed to acclimatize for 20 min before the first indentation commenced. On each skin sample, nine indentation locations were manually selected with use of the built-in microscope of the NanoIndenter XP (MTS Systems, USA). Each location is at least 500 μm away from the others to avoid that measurements influences each other. A new indentation method to determine the mechanical properties of epidermis 55 The top center of the triangles formed by the glyphics, i.e. the primary and secondary lines, is chosen as indentation location to optimize the contact between the indenter and the tissue [Figure 4.2]. All experiments are performed using a sapphire sphere with a radius of 500 μm. The system has load and displacement resolutions of respectively 1 nN and 0.0002 nm. The maximum load depends on the depth limit of indentation, which was set to be maximal 10% of the sample thickness [108]. Preliminary testing demonstrated that this indicates a maximum load of 0.2 mN for stratum corneum and 1 mN for epidermis. The loading/unloading rate was 0.01 mN/s. The maximum load was held for a period of 30 s. The low stiffness of the skin samples required a low surface approach sensitivy and contact stiffness. For both epidermis and stratum corneum, the protocol was repeated on three samples for each subject. Test series were completed within 2 h. Within the laboratory, the temperature and humidity are kept constant at 22°C and 28% RH, respectively. Silicone rubber The skin samples, and especially the stratum corneum samples, are extremely thin (under 20 μm). Measuring such thin samples might be at the limit of the possibilities of the apparatus used. Therefore, to prove the usefulness of the protocol for thin materials, a well-defined homogeneous soft material, the silicone rubber, with different thicknesses was tested with the indentation protocol similar to that for the epidermis. The samples were placed on the substrate without fixation. Indentation locations were pointed automatically, using a 3x3 grid with a distance of 500 μm between the various locations. 1.8 mm Figure 4.2. The top center of the triangles, highlighted by the large red points, formed by the glyphics was chosen as indentation location on the skin samples. 4.1.3 Determination of the Young’s modulus Analytical approach In order to derive a first estimate of the Young‟s modulus, the experimental data of the skin and silicone rubber samples are analysed by the method proposed by Oliver and 56 Chapter 4 Pharr [108], assuming a fully elastic response upon unloading. From the initial unloading slope of the load-displacement (𝑃, ℎ) curve, the reduced modulus Er is obtained: 𝐸𝑟 = 𝜋 𝑑𝑃/ℎ 2 𝐴 (4.1) where 𝐴 is the contact surface. The measured displacement of the tip is in practice hardly ever equal to the contact depth, because at the vicinity of the tip, the surface can sink-in or pile-up (see Figure 4.3). For the special case of frictionless contact of a spherical indenter with a flat linearly elastic half space, the projected contact area 𝐴𝑝 can be calculated for small deformations according to: 𝐴𝑝 = 𝜋𝑎2 = 𝜋(2𝑅 − ℎ𝑐 )ℎ𝑐 (4.2) Subsequently, the Young‟s modulus is calculated following: 1 1 − 𝜈 2 1 − 𝜈𝑖2 = + 𝐸𝑟 𝐸 𝐸𝑖 (4.3) where E and ν are the Young‟s modulus and the Poisson‟s ratio for the specimen and Ei and νi are the same parameters for the indenter. The epidermis, stratum corneum and silicone rubber are considered to be close to incompressible materials, using a Poisson‟s ratio of 0.495. Sink-in Pile-up a hp h a0 hc hp a h hc Figure 4.3: Contact profile developed during indentation where 𝒉 is the indentation depth, 𝒉𝒄 is the contact depth, and 𝒂 is the radius. Obtained from Pelletier et al. [109]. Numerical model To be able to compare the estimated Young‟s moduli via the analytical method, a finite element calculation using MSC.Marc was introduced. An axisymmetric mesh was used to fit the experiments using a Neo-Hookean model, assuming incompressible material behavior. The mesh consisted of 4329 linear quad4 elements, using full integration. The size of the mesh was chosen such that the edges do not influence the stress distribution and contact between the indenter and the sample was assumed to be frictionless. A new indentation method to determine the mechanical properties of epidermis 57 For the silicone rubbers, the Young‟s modulus, 𝐸𝑆𝑅 , was estimated by fitting the average load-displacement curve of the 50 μm thick samples. The value for 𝐸𝑆𝑅 was then used to calculate the unloading curves of the 120 and 2000 μm thick samples. These unloading curves are compared with the experimental data. Since the deformations were small, linear elastic behavior was also assumed for the skin samples. Furthermore, the thickness of the stratum corneum was considered to vary from 10 to 20 μm. The thickness of the viable epidermis was kept constant at 80 μm. First, the Young‟s modulus for the stratum corneum, 𝐸𝑆𝐶 , was derived by fitting the average loaddisplacement curve of the stratum corneum samples. The obtained modulus for stratum corneum, 𝐸𝑆𝐶 , was used to fit the experimental data of the epidermis, such that the modulus for the viable epidermis, 𝐸𝑉𝐸 , could be derived. In order to assess the sensitivity of the fitting approach, the effect of increasing or decreasing 𝐸𝑆𝐶 with a factor 2 on the maximum indentation depth was studied for the epidermis. 4.2 Results Silicone rubber The load-displacement curves obtained from the silicone rubber samples are shown in Figure 4.4. The results were highly reproducible for each thickness. The maximum indentation depth slightly decreases with decreasing sample thickness. Consequently, the slope of the initial unloading curve decreases, which is reflected in the average values for the Young‟s moduli using Oliver & Pharr: i.e. 3.67±0.20, 2.22±0.10 and 1.69±0.04 MPa for a sample thickness of 50, 120 and 2000 m, respectively. From the FE model, the Young‟s modulus was estimated to be 2.16 MPa. When using this values to obtain the unloading curves for the 120 and 2000 m thick sample, it can be shown that the unloading curves and maximum indentation depth for all thicknesses are comparable to the experimental data. Figure 4.4. All force-indentation (𝑷, 𝒉) curves for silicone rubbers with different thicknesses. 58 Chapter 4 Figure 4.5: Fitting curves based on applying a NeoHookean model on the experimental data of the silicone rubbers. Epidermis and stratum corneum An example of the results from one subject is shown in Figure 4.6. Data that significantly displayed measurement errors or deviated from the general response, were ignored. In practice, usually 2 or 3 tests out of a series of 9 meausurements were left out when calculating the average indentation curve (see Table 4.1). Figure 4.7 clearly shows that the average curves are well-overlapping for all subjects. It appears that indentical slopes were obtained for stratum corneum and epidermis. The Young‟s moduli derived via the analytical approach can also be found in Table 4.1. Here, 𝐸𝑆𝐶 is about twice the value of 𝐸𝑆𝐶+𝑉𝐸 . The results of the FE-model are shown in Figure 4.8. For a 20 μm thick stratum corneum and 80 μm thick viable epidermis, 𝐸𝑉𝐸 is identical for 𝐸𝑆𝐶 . Decreasing the thickness of the stratum corneum to 10 μm hardly affects 𝐸𝑉𝐸 . Also increasing in the stiffness of the stratum corneum did not have an effect. As expected, lowering the stiffness of the much thicker viable epidermis causes an increasing indentation depth, from approximately 8 to 12 μm. (a) (b) Figure 4.6. All indentation curves of 1 subject for stratum corneum (a) and epidermis (b). Note that the scales are different. A new indentation method to determine the mechanical properties of epidermis 59 (a) (b) Figure 4.7 Average indentation curves per subject for stratum corneum (a) and epidermis (b). Table 4.1: The number of tests excluded from 9 tests in total and the analytically derived Young’s modulus of all subjects. Stratum corneum Subject # excluded Eanalytical [MPa] 1 3 2.00±0.72 2 2 3.10±2.10 3 3 2.31±0.94 Epidermis # excluded 3 3 1 Eanalytical [MPa] 0.88±0.01 1.07±0.10 1.21±0.38 Figure 4.8. Results of NeoHookean fit on the unloading curves of the epidermis. The thickness of the stratum corneum is varied from 10 (dashed lines) to 20 μm (solid lines). Also the effect of increasing or decreasing the stiffness of the stratum corneum is shown. 4.3 Discussion The major problem in performing indentation experiments on skin is probably the skin‟s surface roughness. In order to have a smooth as possible surface, we used a large spherical indenter (ø=500 μm) such that the contact area was much greater than the diameter of individual cells and also more homogeneous. During preliminary tests that were performed close to the glyphics, it was observed that the poor contact definition in 60 Chapter 4 those areas resulted in an unacceptably high variability per subject. When positioning the indenter at the highest point between a triangle formed by the glyphics, establishing the initial contact between indenter and the tissue was not a problem. In addition, the use of a spherical tip minimizes plastic deformations and stress concentrations and avoid damaging the sample [110]. Using the introduced measurement protocol, highly reproducible data could be obtained for all subjects and the variance between the subjects was negligbly small. In order to obtain reproducible data from an in vitro experiments that are meaningfull, a correct sample preparation is essential. In this study, the epidermal samples were isolated using a dermatome. Although this method does not allow for separating the epidermis at the basal membrane only, its benefit is that the bottom side of the sample with this obtained geometry is in full contact with the substrate. As only small deformations were applied, the results are not influenced by the possible fragments of papillair dermis in the sample. Our tests were performed with epidermis that was thawed and immediately used in a dry environment. As an increasing moisture content in the epidermis decreases the stiffness, it becomes more difficult to define the initial contact surface at higher various humidities in the future. The analytical method of Oliver and Pharr provides an easy method to asses the order of magnitude of the Young‟s modulus from the experimental data. However, the theory holds for materials responding fully elastically upon unloading. In the case of soft tissues, this assumption is far from correct because material responses like piling-up and sinking-in cannot be captured correctly. Due to piling up of the tissue, the projected contact area is bigger then used in the calculations (see Figure 4.3). In our study, the deviation is relatively small, because the use of a large spherical indenter causes a more homogeneous contact surface. The introduction of a numerical model should result into a better approach. The used Neo-Hookean model, however, is also far from correct, but provides a first comparison with the analytical method. The results show that the stiffness of the viable epidermis is comparable to that of the stratum corneum instead of a factor two lower as calculated with Oliver and Pharr. For both epidermal layers, the stiffness of the two layers is approximately 1 MPa, which proves that the viable epidermis considerably contributes to the mechanical response of skin at this lengthscale. In comparison with literature, our values for stratum corneum are on the low side of the published range [101,107,34]. This can be explained by the fact that the local properties studied in literature were mainly determined by the stiffness of individual corneocytes, while our studies focused on the tissue level. In comparison with values for full-thickness skin stiffness from in vivo indentation tests, our values are two orders of magnitude higher [101,111,112]. The mechanical behavior of many soft tissues is described with a multimode Maxwell model. Extending the NeoHookean model into such model would be a logical step forward. However, the relaxationspectrum and corresponding low shear moduli that can A new indentation method to determine the mechanical properties of epidermis 61 be derived from rheological experiments (see Chapter 3) does not influence the fitting on the load-displacement curve. The short relaxtion times that are ranging from 0.002 up to 2 s, are only relevant during high impact loading and are in accordance with the observed small viscoelastic plateau at the maximum applied force in the indentation experiments (see Figure 4.6 and Figure 4.7). Moreover, also a multimode Maxwell model assumes an isotropic material and cannot capture variations in mechanical properties with changes in morphology, composition and moisture content through the epidermis. A better approximation should therefore be an anisotropic model. Then, experimental data from indentation, tensile and shear can be captured using a layered structure. To conclude, the small deformation behavior of epidermis was studied in this study. We have introduced a reliable experimental approach to evaluate the mechanical behavior of epidermal tissue. The results demonstrated that the stiffness of the viable epidermis is comparable to that of the stratum corneum for perpendicular direction at a lengthscale relevant for clinical and cosmetic treatments. The applied load in this study covers the physiologically relevant range. For clinical applications such as transdermal drug delivery, the large deformations and, the ultimate goal, the failure behavior of the epidermal layer needs to be understood. The methods presented in this study are considered to be a suitable tool that can be extended for these purposes. Acknowledgments We would like to thank the plastic surgery department of the Catharina hospital in Eindhoven for providing the skin tissue. Furthermore, we are gratefully to dr. Hagisawa providing the protocol for the histological examination. Chapter 5 Linear viscoelastic behavior of subcutaneous adipose tissue The content of this chapter is based on M. Geerligs, G.W.M. Peters, P.A.J. Ackermans, C.W.J. Oomens, and F.P.T. Baaijens (2008), Linear viscoelastic behavior of subcutaneous adipose tissue, Biorheology; 45(6): pp 677-688. 64 Chapter 5 5.1 Introduction The mechanical behavior of subcutaneous adipose tissue, also called hypodermis, is a widely ignored topic in the biomechanics literature. A plethora of papers can be found on properties of skin and skeletal muscle, but only few papers have addressed the properties of the layer in between [38,43,39,113,114]. This is noteworthy, because adipose tissue plays an important role in the load transfer between different structures in the body during breathing, body movements or exercise, or when exposed to therapeutic stretching during physiotherapy and massage. It is well recognized that the subcutaneous fat experiences larger strains than the dermis during suction and that its stiffness is likely to be a few orders less than that of the dermis [1,115]. However, it is still not common practice to take the adjacent adipose layer into account when the combined mechanical behavior of skin, fat and muscle tissue is modeled. Currently it would be difficult to do so, because values for mechanical parameters of adipose tissue are limited and inconsistent in the literature. Thus, there is a need to develop a parametric and constitutive model of subcutaneous adipose tissue, which can be implemented in numerical models of the whole skin as well as in multilayer models including skin, fat and muscle. Numerical models including the subcutaneous fat layer are needed in a wide field of applications, e.g. studying skin device contact, needle insertion procedures and the removal of skin adhesives. Rheological experiments are accepted to be a good starting point to develop such a constitutive model. For a meaningful interpretation of the mechanical behavior of the adipose tissue, it is essential to know the tissue composition. The present paper is focused on subcutaneous adipose tissue, which is a type of connective tissue throughout the body found between the dermis and the aponeurosis and fasciae of the muscles. However, the fat pads on the palm of the hand and foot are considered to be different, since they contain a much higher ratio of unsaturated versus saturated fatty acids and are therefore morphologically different. Relatively small differences in tissue composition exist at the other body sites. Subcutaneous adipose tissue is a loose association of lipid-filled cells called white adipocytes, of which 90-99% is triglyceride, 5-30% water and 2-3% protein. Lipids within the white adipocytes are organized in one droplet. The diameter of the white adipocytes ranges from 30 to 70 μm, depending on the site of deposition [17]. Collections of white adipocytes comprise fat lobules, each of which is supplied by an arteriole and surrounded by connective tissue septae. Each adipocyte is in contact with at least one capillary. In healthy adults, only one third of the subcutaneous adipose tissue contains mature adipocytes [17]. The remaining two thirds consists of blood vessels, nerves, fibroblasts, and adipocyte precursor cells. The subcutaneous adipose tissue of the lower trunk and the gluteal-thigh region is further divided into two distinct layers: the superficial and deep subcutaneous adipose tissue [116,19]. Both morphological and metabolic differences were found between those two layers [116,117,118], but it is not clear if these layers differ in terms of the mechanical properties. Linear viscoelastic behavior of subcutaneous adipose tissue 65 To our knowledge, only a few authors studied the mechanical properties of the subcutaneous adipose tissue. Of those, focus has been associated with breast tissue, particularly in the early detection of cancerous tissues [95,44,119,119,120,41,43]. These studies have generally utilized indirect and non-invasive measurements. The largest study involving 70 samples of breast fat tissue using ex vivo indentation experiments yielded a mean Young‟s modulus of 3.21 kPa [19]. Linear viscoelastic behavior was shown up to 50% strain during uniaxial tension for abdominal subcutaneous tissue of rats when applying incremental displacement steps of 1 mm followed by a 1 second relaxation period [113]. Patel et al. [39] measured the storage and loss moduli of subcutaneous fat tissue, also from the abdomen, for strains up to 20%. The results showed a frequency-dependent shear moduli decreasing, which decreased with increasing strain. These data, however, involved measurements outside the linear viscoelastic strain range. Recently, the mechanical behavior of subcutaneous adipose tissue of the buttock was measured in relation to pressure ulcers by performing confined compression tests, but no mechanical parameters for modeling could be derived from the results [121,114]. All the above-mentioned studies only give limited descriptions of the mechanical behavior, either because the focus was only on the differences between breast tissue types, or on long term quasi-static behavior [113,121], or because the authors were only interested in a comparison of properties between human fat and a mimicking material [39]. Our ultimate goal is to develop a skin model that includes the mechanical properties of all skin layers separately, and can be used in a numerical model. Since it may be predicted that the mechanical behavior of adipose tissue contributes considerably to the overall skin behavior, there is a need to develop a thoroughly tested constitutive model describing the mechanical behavior for large strains. The formulation of such a model will be based on rheological experiments in vitro. The first step is to investigate the material bulk properties within the linear viscoelastic strain region, which is defined as the range of strain amplitudes where the material properties are independent of the applied strain. The types of experiments are relatively simple to perform and hence, it is appropriate to design experimental procedures as well as to identify experimental problems. The linear viscoelastic parameters obtained will form the basis for a non-linear viscoelastic model in future work. The concept will be developed for porcine subcutaneous adipose tissue because of the availability and minimal biological variability among specimens. The objective of the current study is to use dynamic mechanical thermal analysis (DMTA) in combination with Time Temperature Superposition (TTS) to determine the small oscillatory strain behavior of subcutaneous adipose tissue in vitro. DMTA is performed through oscillatory shear experiments up to 100 rad/s at various temperatures. Next, the linear viscoelastic power-law memory function, commonly used for soft-solids, will be introduced to describe the small strain viscoelastic behavior of this tissue. 66 Chapter 5 5.2 Methods and Materials 5.2.1 Sample preparation Porcine subcutaneous fat tissues were obtained from a local slaughterhouse (Ballering, Son, The Netherlands), where they were cut into transverse slices of 1.5-2.0 mm thick and stored at 4°C. In porcine species, the back fat is divided in an outer, middle and inner layer of subcutaneous tissue because the adipocyte features of these layers differ with respect to size, number and metabolic activity. The porcine middle layer, which is used in the present study, is comparable to the deep subcutaneous layer in the abdominal region of humans [122]. All pigs were Landrace, having a dressed carcass weight of approximately 83 kilograms, and were 14-18 weeks old at necropsy. Within 48 hr of collection, circular tissue samples were obtained from the slices with an 8 mm diameter cork borer. Next the samples were stored ice-cooled in a PBS solution and tested within the subsequent 4 hours. An overview of the number of specimens and the number of samples from each specimen per test is given in Table 5.1. Methods of tissue preservation may change the mechanical properties of tissue due to changes in tissue quality [85]. Rapid freezing, which has not been demonstrated to change the fatty acid composition compared to fresh tissues [123], is an attractive solution for storing tissue for prolonged periods. Thus, in order to assess whether snap freezing preserves mechanical properties, adipose tissue was snap-frozen by immersion in 2-methylbutane cooled by liquid nitrogen and stored at –80°C until use for mechanical testing. Thawing of the samples was done slowly within an ice-cooled box. In order to assess these storage conditions, histological sections were examined by light microscopy. For that, the specimens were fixed in 10% phosphate-buffered formalin and processed for conventional paraffin embedding. The specimens were cut into 5-μm thick sections and stained with hematoxylin and eosin (H&E). Since all lipids were extracted out of the adipocytes by using the conventional paraffin embedding technique, other specimens were embedded in O.C.T. compound (TISSUE-TEC) and frozen for lipid staining. These specimens were cut into 8-μm thick sections at –20°C, stained with oil Red O (Sigma) and counterstained with hematoxylin. 5.2.2 Rheological methods To determine the linear viscoelastic properties, oscillatory shear experiments were performed using a rotational rheometer (Advanced Rheometric Expansion System (ARES), Rheometrics Scientifc, USA) with a controlled strain mode, and parallel plate geometry in combination with a Peltier environmental control unit. Sand-blasted plates were used to prevent slippage. An oscilloscope was used to ascertain that the shape of the torque signal was indeed sinusoidal. Samples were compressed between the plates by lowering the upper plate until an axial force of 0.1 g was reached. Linear viscoelastic behavior of subcutaneous adipose tissue 67 In the experiments a sinusoidal strain γ(t) insteady state and within the range of linear viscoelastic behavior, resulted in a sinusoidal shear rate, g(t), and shear stress,τ(t) with a phase shift δ: (t ) 0 sin(t ) , (5.1) (t ) Gd 0 sin(t ) . (5.2) The dynamic shear modulus Gd(ω,T) and the phase shift δ(ω,T) are both a function of the angular frequency ω and temperature T. It is common to separate the dynamic shear modulus into a storage modulus, G', representing the elastic behavior since this describes the stress in phase with the strain, and a loss modulus, G'', representing the viscous behavior, 12 out of phase with the strain, i.e. in phase with the strain rate: Gd G' 2 G"2 . (5.3) The phase shift d to (5.3: tan( ) G" G' . (5.4) The Time-Temperature Superposition (TTS) principle is applicable when data can be shifted to and from a reference temperature T0 to form a master curve [124]. The advantage of this principle is that the frequency domain can be extended beyond the measurement limits as well as that data can be shifted to other working temperatures. A smooth master curve is obtained by shifting frequency sweep curves obtained at different temperatures horizontally and vertically on the curve obtained at the reference temperature, until all the curves overlap. Normally the horizontal shift factor aT is applied to the phase angle δ. Subsequently, the dynamic shear modulus Gd, and also G' and G'', can be shifted along the horizontal and vertical axis to a reference temperature with the horizontal shift factor aT and a vertical shift factor bT: tan (, T ) tan (aT , T0 , Gd ( , T ) 1 Gd (aT , T0 ). bT (5.5) (5.6) 68 Chapter 5 5.2.3 Testing procedure Test protocols were based on measuring the linear viscoelastic properties of other soft biological tissues, such as brain [125], muscle [126] and thrombus [93]. The linear viscoelastic regime was determined using oscillatory shear experiments with constant frequency and varying strain. Strain sweeps were performed from 0.04% to 10% at frequencies of 1, 10 and 100 rad/s and 20°C. A constant strain within the determined linear regime of 0.1% was chosen for the subsequent frequency sweep tests. The frequency sweep was repeated three times to avoid tissue conditioning phenomena, observed during preliminary testing. We did not carry out traditional preconditioning. Instead we performed always three frequency sweeps, increasing the frequency stepwise logarithmically from 1 to 100 rad/s and then performing the data analysis on the third frequency sweep. This protocol was also used to examine the influence of snap freezing and thawing on the mechanical properties of subcutaneous fat tissue. For this purpose, samples from 3 pigs were tested, both fresh and after freezing and thawing. All tests were performed at 20°C. To investigate whether the TTS principle is applicable to subcutaneous adipose tissue, frequency/temperature sweeps were successively performed at temperatures of 5, 20, 35 and 40˚C, at 0.1% strain and frequencies ranging from 1-100 rad/s. Again, two successive frequency sweeps from 1-100 rad/s were performed prior to these frequency/temperature sweep tests. The temperature range is bounded at the low end by the phase transition temperature of water and above by temperatures at which protein degradation is likely to occur. To check the possible influence of the order of heating or cooling, 3 samples were also subjected to a frequency/temperature sweep with decreasing temperatures. As a control for the applied power-law model, a stress relaxation experiment additional to the frequency sweep tests was performed. In these experiments a step strain of 0.1% was applied during 100 s. Table 5.1: Overview of number of specimens and number of samples per specimen used for the experiments. Test Strain Sweep Frequency Sweep 5x repeated Model fit Effect snapfreezing Frequency/Temperature Sweep Increasing T Decreasing T Stress relaxation * sample number per condition # Specimens (# samples per specimen) 3 (4,5,3) 3 (3,3,3) 3 (3,3,3) 3 (3,5,3)* 2 (3,3) 2 (2,1) 1 (5) Linear viscoelastic behavior of subcutaneous adipose tissue 69 5.2.4 Statistics For the strain sweep, frequency sweep and stress relaxation tests with fresh tissue, the average values and standard deviations were calculated for the mechanical parameters at different testing strains or frequencies. In order to determine whether snap-freezing has a significant effect on the mechanical parameters, data were analyzed with the linear mixed model [127] by using the software Splus. For this purpose, the log of the frequency sweep data was used. The linear mixed model was chosen because it accounts for biological variability among samples and among specimens while analyzing freezing effects. 5.3 Results 5.3.1 Small oscillatory strain behavior Fig. 1 shows the results for the strain sweep tests at 10 rad/s for both G' and G''. Both moduli and phase shift, which is not shown here, were found to be nearly independent of strain for amplitudes up to 0.1%. Tests at other frequencies revealed similar results and are therefore also not shown. Preliminary testing showed that tissue conditioning phenomena are minimised by performing two frequency sweeps before the actual measurement (Figure 5.1). Results for the storage and loss moduli and the phase angle, as functions of the applied frequency, are shown in Figure 5.2. The biological variation appeared to be small. Taking all samples from fresh specimens together, the shear modulus Gd is found to be 14.9 kPa ± 4.8 kPa at 10 rad/s. The average phase angle is approximately 21.0° over all frequencies, indicating that the complex modulus is dominated by elastic behavior. Results of stress relaxation are depicted in Figure 5.3. The shear modulus decreases over a decade over 100 s. Figure 5.1: Results from strain sweep tests. Average G’ and G” demonstrate a linear viscoelastic regime up to 0.1% strain at a frequency of 10 rad/s. 70 Chapter 5 Figure 5.2. Frequency sweep results: (a) mean G’, and G”, the standard deviations and the fitted model; (b) mean δ , standard deviation and the estimated fit. Figure 5.3. Stress relaxation behavior. 5.3.2 Model application The shear stress response for linear viscoelastic behavior is usually described in terms of the Boltzmann integral: t G (t t ' ) (t ' )dt ' , (5.7) where G(t) is the relaxation function and is the shear rate. The results of the frequency sweeps indicate that a power-law relation can adequately describe the storage and loss moduli: Linear viscoelastic behavior of subcutaneous adipose tissue 71 G' () G' (1) p , (5.8) with G'(1) and p as constants [128]. The same relation is used for G''. The phase angle can be expressed in terms of the exponent p [128,129]: tan G" p tan G' 2 . (5.9) So the small oscillatory strain behavior is captured by an approximation with only two constants (p,G(1)). It is known [128] that the relaxation function G(t) in Eq. (5.7 can be written as G(t ) G(1)t p . (5.10) The constants G(1) is related to G'(1) by G (1) 2G ' (1)( p!) p sin , p 2 (5.11) where p! is the factorial function. The expressions for G' and G'' were fitted simultaneously, resulting in one value for p per sample. Next, the exponent p was used to calculate the phase angle corresponding to the frequency sweeps (Figure 5.2) and the relaxation modulus for the stress relaxation experiments (Figure 5.3). In all cases, the exponent p was in the range from 0.18-0.25, with a mean value of 0.21. 5.3.3 Time-Temperature Superposition Results of the frequency/temperature sweeps show that the phase angle is not dependent on temperature for increasing temperature (data not shown). However, the shear modulus Gd can be shifted along the horizontal frequency axis to obtain a smooth master curve at a reference temperature of 20°C (Figure 5.4), in such a way that Gd (, T ) Gd (aT , T0 ) . Results of the frequency/temperature sweeps with decreasing temperature were similar to those with increasing temperature and are therefore not shown here. The curves of Gd for different temperatures show curves that overlap extensively such that the frequency domain could be extended to almost 3 decades (Figure 5.4). The horizontal shift factors, as a function of the temperature at which each dataset was acquired, can be captured reasonably well with an exponential function with a quadratic power: aT e aT0 2 bT0 c , (5.12) 72 Chapter 5 with a = -0.0046 ± 0.0021, b = 2.54 ± 1.25 and c = -351.39 ± 183.39 (Figure 5.5). From this, it can be calculated that Gd at body temperature is approximately 7.5 kPa at 10 rad/s. Figure 5.4. (a) Example of frequency sweeps performed at different temperatures, which can be shifted horizontally; (b) master curve of Gd obtained for two specimens each within 3 samples. Figure 5.5. Shift factor aT versus temperature T. Experimental data from three sepcimesn (,○,□) from two specimens are shown together with the mean fit. 5.3.4 Freezing effects From the histological sections, severe damage could be observed in 2 out of 12 samples. Either cells were less packed or cell membranes were ruptured (Figure 5.6). However, less or no damage occurred when tissue was embedded in the O.C.T. compound. So it remains unclear, whether the damage was only due to the snap freezing method and/or preparation artefacts. The frequency sweeps showed that the differences of the intercepts of regression lines were not statistically different, whereas the differences in the slopes of the lines for G’ were statistically different (Figure 5.7). However, the biological variance among all samples is larger than the difference between the fresh and snap frozen samples. This can Linear viscoelastic behavior of subcutaneous adipose tissue 73 be seen in Figure 5.7, where the regression line of the frozen samples lies within the biological variation of the fresh samples. So from a practical viewpoint, the observed difference of slopes for the two conditions is negligible for G'. In the case of the G'' slopes, there was no statistical difference. Taken this all together means that snap freezing does not show any effects on the mechanical properties compared to fresh tissue. Figure 5.6. (a) Fresh adipose tissue, (b) adipose tissue after snap freezing without damage, (c) tissue damage after snap freezing. Figure 5.7. The biological variation on the slope of the normalized regression lines of G’ is shown. The dotted lines represent the limits of two times the standard deviations on both sides of the belonging regression line. 5.4 Discussion The results indicate that the shear moduli can be shifted to measurement conditions described in the literature when using the Time-Temperature Superposition. From the literature it is known that the linear region for other soft-solids consisting of loosely bounded soft particles is below 1%, which is consistent with the present observations. In fact, the linear region is considered to be only up to 0.2% strain. This small strain was the maximum strain that could still represent linear behavior within an acceptable signalto-noise ratio. Too large strain amplitudes are outside the linear strain regime and reduce the “apparent” modulus, which might explain the difference with Patel‟s data [39]. In 74 Chapter 5 comparison with Samani et al. [43], who applied a quasi-static loading with a frequency of 0.1 rad/s resulting in a Young‟s modulus of 3.2 kPa., our shear modulus Gd(ω = 0.1 rad/s, T = 20°) is 5.6 kPa, which results into a higher Young‟s modulus. In addition, the present results show an obvious temperature dependency and a specific start-up behavior. The reasons for these differences are unknown. The reproducible long term variations in the beginning of a frequency sweep, a change in the slope of G', are not yet understood. Snap freezing may cause tissue damage resulting in less packed cells or ruptured membranes, but it is more likely that the observed artifacts are caused by the chosen histological technique. Snap freezing did not appear to have an effect on the mechanical behavior. Although the slopes of the regression lines for G' demonstrated significant differences, the observed difference is smaller than the biological variation between samples. Many of the environmental conditions, other than temperature, are difficult to control. Since the snap frozen samples were measured on separate days to the fresh samples, the environmental conditions might have influenced the measurement outcomes per specimen. In the present study porcine tissue from the slaughterhouse was used. The nature of the source of biological material at the present study was such that biological variation between specimens was relatively small. The adipocytes of the pigs had a diameter of 70 μm or greater whereas that of human adipocytes varies from 30 to 70 μm. The question arises whether other tissue composites contribute more to the mechanical behavior of the bulk tissue than the adipocytes. Besides blood vessels and the collagen fiber network, no other significant composites are present in the adipose tissue. Tissue with visible blood vessels was excluded from testing. Therefore, it is conceivable that the stiff collagen fiber network surrounding the fat lobules plays an important role in the overall mechanical behavior. To our knowledge, it is the first time that this common rheological model has been applied to biological soft tissue. The power-law model fits the experimental data well. The p-values obtained are comparable to those of other soft materials in the literature. It should be noted, however, that the fit on the slope of the stress relaxation behavior could be improved although an optimization process would not yield any further benefit. More interesting is the fact that we have introduced a model that can be extended to a threedimensional non-linear model capturing large deformations with the possibility to include the build up and breakdown behavior of initial structures. Nevertheless, experiments in the non-linear strain regime are necessary to prove whether or not this promising model can fit those predictions. Also, Time-Temperature Superposition is applicable to this type of biological tissue. Mechanical properties measured at any temperature can be shifted to body temperature by applying the Time-Temperature Superposition. However, the applicable temperature range for experiments is physically bound by phase transitions at low temperatures and the solidifying of proteins above 41°C. The measurements already showed a much larger variation at the upper limit of the temperature range, i.e. at 40°C, than at any other Linear viscoelastic behavior of subcutaneous adipose tissue 75 temperature. This indicates that it is recommended to avoid this boundary of the temperature range. Chapter 6 Does subcutaneous adipose tissue behave as an (anti-)thyxotropic material? The contents of this chapter are based on M. Geerligs, G.W.M. Peters, P.A.J. Ackermans, C.W.J. Oomens, and F.P.T. Baaijens (2008), Does subcutaneous adipose tissue behave as a thyxotropic material?, Journal of Biomechanics, accepted. 78 Chapter 6 6.1 Introduction The mechanical load transfer from a skin contact area to deeper tissues involves several tissue layers. On most body sites, the subcutaneous adipose tissue considerably contributes to this load transfer. However, when numerical models are used to predict the stress response due to external loading, the focus is either on the skin-device contact or on the deeper tissue layers while the subcutaneous fat layer is often ignored. This omission might be related to the lack of defined parameters, which describe the mechanical behavior of adipose tissues. This is particularly surprising given the critical roles for adipose tissues in the medical and cosmetic fields, involving, for example, implantable drugs delivery, skin adhesive removal, deep tissue injury and needle insertion procedures. Recently, our previous work on the linear behavior of subcutaneous adipose tissue has shown that the linear strain regime is valid for very small strains only, i.e. 0.001 [130]. In most applications, however, much higher deformations occur in the adipose tissue for prolonged periods. Indeed, for wheelchair or bedridden patients, for example, this might lead to the development of deep tissue injury under bony prominences within a time frame of minutes to hours, during which stress relaxation in the compressed tissue might occur [40]. Numerical models based on experimental data are of indispensable value to predict the onset and progression of such mechanical-induced damage. Currently, there is a paucity of papers on the mechanical properties of the subcutaneous adipose tissue found beneath hairy skin. Viscoelastic properties of single human adipocytes have been recently characterized using AFM resulting in a relaxed modulus and relaxation time for either load or deformation [131]. Few related in vitro studies on tissue behavior exist. Of these, rheological measurements demonstrated a decrease in viscosity with increasing shear rate [39]. In addition, the authors suggested that adipose tissue loses firmness with increasing strain and frequency, a state which is not recoverable. In a separate study, ovine subcutaneous tissue was subjected to ramp-andhold cycles during confined compression tests at various ramp rates [39,40]. The results were given in the form of a transient aggregate modulus and short-term elastic moduli. They also found a strong deformation rate dependency. Short-term moduli were in the order of 20 kPa. In an alternative in vivo approach, a suction device yielded experimental parameters which, when combined with numerical modeling, led to a first estimation of non-linear material parameters for human skin [115]. To our knowledge, there are no in vivo studies considering subcutaneous adipose tissue as a single layer. By contrast, some in vivo studies have examined the mechanical properties for a compliant system consisting of skin and subcutaneous adipose tissue [121,132]. The work mentioned above describes a range of loading conditions, often combining techniques involving indentation, confined compression, stress relaxation and constant shear responses. Clearly, this makes comparison of data from the studies problematic in Does subcutaneous adipose tissue behave as an (anti-)thyxotropic material? 79 nature. For a general constitutive model for adipose tissue a more systematic approach is required. The material structure of subcutaneous adipose tissue does not relate conveniently to other biological tissues. Its main component is the white adipocyte. The remaining components are water (5-30% weight) and protein (2-3% weight). The white adipocytes are filled with a large fat droplet imposing forces on both the nucleus and the small cytoplasmic volume at the cell periphery. The composition of the white adipocytes depends on the specific function and body site. As an example, differences throughout the human body are known for the proportions of saturated fatty acids, monosaturated versus polysaturated fat and the lipolysis rate [17]. White adipocytes are collected in a surrounding fiber network. The adipose tissue is well-vascularized throughout with each adipocyte in contact with at least one capillary. Hence, adipose tissue is susceptible to ischemia and hypoxia, which influence its mechanical response. Our previous work on the small strain behavior of adipose tissue has shown that reproducible results are obtained in an in-vitro set-up using a rheometer with parallel plate geometry and that the behavior can be described with a power-law model [130]. However, sometimes tissue samples were found to be much stiffer than the mean value and early work at higher strains has suggested that (reversible) structural changes start to play a role. In addition, earlier large strain studies formed the incentive for a more systematic approach at higher strains to elucidate the phenomena that havealready been described. Therefore, the present study aims to provide systematic data for long-term small strain behavior as well as the effect of strain history, with the purpose of contributing to the development of a constitutive model. Accordingly, the work is divided in two parts. The first part contains long term oscillatory tests at small strains to investigate temporal effects of the adipose tissue samples. Subsequently, strain-dependency tests, comprising constant shear, stress relaxation and constant strain rate, are applied. From these tests, non-linear parameters can be obtained useful for constitutive modeling. Such an experimental approach is designed to gain insight on the mechanical response of adipose tissue under shear where the effect of strain history, strain level and duration is taken into account. 6.2 Materials & Methods 6.2.1 Sample preparation In porcine species, the subcutaneous fat layer on the back is divided in an outer, middle and inner layer. The porcine middle layer was selected for use, as it is considered to be the most comparable with the deep subcutaneous layer in the abdominal region of humans [122]. The tissue was obtained from a local slaughterhouse, where they were cut into transverse slices of approximately 1.5 mm thick. In our laboratories, circular samples were obtained from the slices with an 8 mm diameter cork borer. The samples 80 Chapter 6 were stored in a Phosphate Buffered Saline solution (PBS) in ice-cooled boxes and tested within 48 hr of collection. If measurements were repeated after a certain period of recovery, each sample was stored in PBS between measurements. All pigs were Landrace, having a dressed carcass weight of approximately 83 kilograms, and were 1418 weeks old at necropsy. 6.2.2 Rheological methods All experiments were performed on a rotational rheometer (ARES, Rheometric Scientific, USA) with parallel plate geometry in combination with a Peltier Environmental control unit and a fluid bath. Plates were sand-blasted to prevent slippage. The upper plate was lowered to compress the sample until the sample experienced an axial force of 1 g. All loading protocols, which were based on previous experiments on soft biological tissues (Van Dam, 2008; Hrapko, 2006), are summarized in Figure 6.1. Long-term dynamic behavior within the linear viscoelastic regime was studied with time sweep tests (Figure 6.1a). Tests were performed at a frequency of 10 rad/s with a strain amplitude of 0.001 at body temperature (37°C), lasting at least 45 minutes. The chosen strain amplitude was previously determined to be the maximum strain within the linear viscoelastic regime [130]. Time sweeps were repeated after various time periods of recovery, namely 0, 0.5, 1 and 3 hours. Shear experiments in the non-linear regime were preceded by two successive frequency sweeps with a frequency of 1-100 rad/s and a strain amplitude of 0.001. This procedure was adopted to minimize the effects of pre-conditioning [130]. Subsequently, the sample was tested in either a series of constant shear rate experiments, constant shear experiments or stress relaxation experiments (Figure6.1b-e). The measurement protocols were based on previous experiments on soft biological tissues. Constant shear rate experiments with various strain amplitude were designed to investigate any potential damaging effect in the mechanical behavior due to the previous strain history on the immediate mechanical response. The first series of sequences were loading-unloading tests conducted with a constant shear rate of 1 s−1 and strains incrementally increasing from 0.01 up to 0.5 (Figure 6.1b). The sample was left to recover at zero strain for at least 10 times the loading time after each loading-unloading cycle. In total, 20 cycles were applied. In another series of sequences with the same constant shear rate, strains were applied in decreasing order (Figure 6.1c). Again the sample was left to recover at zero strain for at least 10 times the loading time after each loading-unloading cycle. In order to investigate possible reversible changes, this sequence was repeated after 0, 1 and 3 hours of rest. The next set of experiments was designed to apply constant shear at increasing shear rate (Figure 6.1d). Loading-unloading cycles were conducted with constant shear rate increasing from 0.01 s−1 to 1 s−1 per cycle with maximum strain amplitude of 0.15. Does subcutaneous adipose tissue behave as an (anti-)thyxotropic material? 81 Between two cycles, the sample was again left to recover for at least 10 times the loading time. Finally, stress relaxation experiments were composed of a series of ramp-and-hold tests at different strain levels (Figure 6.1e). During the loading and unloading phase, a constant strain rate of 1 s-1 was imposed. The maximum strain was held for 10 s during which the relaxation of the material was recorded. The sample was left to recover for a period of at least 100 s during which time the tissue response was monitored. The test was repeated for four different strain levels, namely 0.01, 0.05, 0.1 and 0.15. An overview of the number of specimens and the number of samples from each specimen per test is given in Error! Reference source not found.. Figure 6.1: Schematic illustration of test sequences. (a) Time sweep tests; (b) Constant shear rate experiments with increasing shear strains; (c) constant shear rate experiments with decreasing shear strains; (d) constant shear experiments with increasing shear rate; (e) stress relaxation experiments. 82 Chapter 6 Table 6.1: Overview of number of samples used for the experiments. Test Time sweep Constant shear rate increasing shear decreasing shear Constant shear Stress relaxation # specimens (# samples per specimen) 4 (1,6,4,2) 1(3) 1(2) 2(3,3) 2(3,3) 6.3 Results 6.3.1 Long term small strain behavior An interesting qualitative trend was observed during the time sweep experiments (Figure 6.2a). The samples showed a gradual increase of both initial storage modulus and initial loss moduli over time from the start of the experiment. However, after a period, a rapid increase in stiffness, G‟, occurred in all samples indicating a change in tissue structure. The moduli showed a further slight increase until a steady state was reached. During the steep increases the moduli increased by a range of roughly 1.5-15 kPa. The rapid stiffening occurred at some time between 250 s and 1200 s. An overview of the stiffness increase and start time for all 13 samples is given in Figure 6.2b. Figure 6.2: (a) Typical result of a time sweep: the arrow indicates the measured increase in the storage modulus G’ during quick stiffening phase (ΔG’). (b) ΔG’ against the start time of the stiffening for samples from all specimens. Experiments with repeated time sweeps show that the material behavior is reversible, although recovery takes several hours to complete (Figure 6.3). To enable comparison between specimens, the shear moduli of each specimen were normalized to a scale r from 0 to 1, e.g. from the initial modulus up to the final steady state level of the initial test. When the second time sweep is immediately performed after the first time sweep, Does subcutaneous adipose tissue behave as an (anti-)thyxotropic material? 83 the initial moduli remains constant at the plateau value, see Fig. 3a. After a recovery period of 1 hour, the initial value for the moduli is reduced, although not reaching the level corresponding to that during the first time sweep. After 3 hours the material appeared to be totally recovered and a qualitatively comparable curve could be obtained. A third test on the same sample after a further 3 hours of recovery (trest =6 hr in Figure 6.3b) demonstrated a qualitatively similar curve. Figure 6.3: Repetition of time sweeps. (a) The shear moduli are scaled from 0 to 1, from the start value of the initial test on the specific sample up to the stationary state at the higher plateau. The initial response from one sample is shown here by the thick line; the other lines represent the response after various periods of rest time for the same sample; (b) A sample is loaded again after 3 and 6 hours of rest to demonstrate the reversible behavior. 6.3.2 Large strain experiments In the constant shear rate experiment with increasing strains (Figure 6.4a), three phases can be distinguished as delineated by strain values of 0.15 and 0.30 in Fig. 4b. If the stress strain curve (Figure 6.4b) is enlarged to highlight the first phase, it is evident that the responses at strains up to 0.15, within reasonable limits, overlap (Figure 6.4c) and can be considered to be reproducible. For strains above 0.15, however, the loading curves are changing. For increasing strain, the stress is decreasing for subsequent loading cycles indicating strain induced changes in the tissue. By contrast, above 0.3 strain, the curves appear to overlap for repeated load cycles suggesting that tissue structure is not changing further. Although the stress response greatly differs for the three phases for the large strain range, the stress response within the linear strain region did not change. The results of the constant shear rate experiments with decreasing strain are depicted in Figure 6.5. Notice that the tissue structure immediately changed in the first cycle, and that the subsequent loading cycles followed the first curve. In addition, despite applying strains of approximately 0.3, the specimens were able to recover after a sufficient recovery period. 84 Chapter 6 Figure 6.4: Average results from constant shear rate experiment with increasing strain amplitude. (a) Applied shear strain with reproducible strain rate; (b) the three different phases of the stress-strain response; (c) stress-strain response up to 0.1% strain. Figure 6.5: Average results from constant shear rate experiment with decreasing strain amplitude. (a) Applied shear strain with reproducible strain rate; (b) Stress-strain curves from constant shear rate experiments with decreasing strain. The applied sequences have been repeated after various rest periods (dotted lines). Constant shear rate experiments with increasing strain rate were applied up to a maximum strain of 0.15. From the results it can be observed that the stress as a function of strain is strain rate dependent and that the response stiffens with increasing strain rate for both the linear and non-linear range (Figure 6.6). Results of the stress relaxation experiments are illustrated in Figure 6.7. The results show practically overlapping curves for the loading phase in the linear strain regime (Figure 6.7). The stress response in the non-linear strain region followed a nearly identical curve for each sample (Figure 6.7c). During stress relaxation, the relaxation modulus did not reach yet a plateau value within the relaxation time allowed (Figure 6.7d). The averaged relaxation modulus decreases as a function of applied strain, where the difference becomes smaller for larger strains. Does subcutaneous adipose tissue behave as an (anti-)thyxotropic material? 85 Figure 6.6: Constant shear experiments with increasing strain rate. Figure 6.7: Results of stress relaxation experiments in shear (test sequence C). (a) stress vs. time for one sample; (b) stress-strain response for one sample; (c) peak stress variations (n=6); (d) average relaxation modulus vs. time. 6.4 Discussion For this study, both long term behavior at small strains and strain history effects at large strains were investigated. Samples from porcine subcutaneous adipose tissue 86 Chapter 6 demonstrated noteworthy behavior for both types of loading. The long term behavior obtained at small strains is qualitatively reproducible. However, in quantitative terms, both the time of onset and the amount of increase in moduli values varied considerably (Figure 6.2b). The cause for those variations is not yet understood. Nevertheless, the observed sudden stiffening of the material up to a decade is crucial for understanding and measuring the material behavior of adipose tissue. The rapid increase in tissue stiffness implies structural changes, which are reversible, and might influence mechanical testing over longer time periods. Responses in the large strain regime were examined initially by performing constant shear rate experiments (Figure 6.1b). The stress-strain response changed for increasing strains and can be divided in three phases (Figure 6.4b). Material behavior changed dramatically. Additional experiments were therefore performed to ratify the tissue structure changes due to mechanical loading, as well as to investigate tissue recovery. These experiments with decreasing shear confirmed that the stress-strain response is dependent on the strain history. The applied large strains here are in accordance with physiologically relevant strains, for example equivalent to that estimated during sitting [121]. From the constant shear rate experiments it can be concluded that up to 0.15 strain, the adipose tissue might behave mechanically similar to other biological tissues such as brain tissue and thrombus [125,133,92]. Because tissue structure changes might occur above 0.15 strain, the subsequent large strain experiments were performed up to this limit. The constant shear experiments and stress relaxation tests indicate both reliability and reproducibility of the test method and show similar trends as those reported for samples from brain and thrombus tissues. These findings therefore support the appropriateness of a Mooney-Rivlin like model for the simulation of the first phase of large strains. Structural changes due to mechanical loading are an indication of thixotropic behavior. Thixotropic behavior is defined as a time-dependent decrease of viscosity or modulus induced by deformation which is a reversible effect when the deformation is removed [134]. When the deformation causes a reversible, time-dependent increase, it is called antithixotropy. (Anti-)thixotropic materials may or may not be viscoelastic in nature. Both the long term behavior at small strains and the constant shear rate experiments indicate reversible structural changes. However, the small strain results indicate an antithixotropic behavior, while the large strain results show a thixotropic behavior that is observed at the large strain only. The stress relaxation response evidently indicates viscoelastic behavior. In the human body, blood and synovial fluid are known to behave thixotropically [135,136,134]. For adipose tissues, it would be interesting to visualize using a confocal microscope to see whether adipocytes and/or the surrounding collagen network behavior rearrange with mechanical loading. In addition, to examine the mechanical behavior for strains above 0.15 specific test methods are needed, as summarized in a recent overview [134]. When establishing such experiments, the large Does subcutaneous adipose tissue behave as an (anti-)thyxotropic material? 87 strain behavior of adipose tissues should be studied preferably before stiffening occurs at small strains to be independent of time effects. The outcome of our large strain studies were not influenced by time effects. From the large deformation studies, the experiment with an increasing strain up to 50% represented the most prolonged lasting approximately 2300 s, including the preceding frequency sweeps. The loading-unloading cycle was maintained at a maximum strain for only 1 s, which amounted to only 20 s in total. The duration of the other experiment with increasing strains was less than 500 s. The increasing shear rate experiments and stress relaxation experiments lasted approximately 900 and 750 s with short term loadingunloading cycle as well. So the long-term time effects did not influence the outcome of the strain-dependency studies. The observed reversible behavior is in contradiction with a previous study [39]. These authors argue that even at small deformations human adipose tissue is not able to recover during creep tests. Since the linear strain regime is only applicable to very small strains, it might be that those measurements are performed outside this region or that the recovery time was insufficient. The described phenomena may have major consequences for the interpretation of results of biomechanical studies. A field of interest of the authors is the development of pressure ulcers, tissue degeneration after prolonged loading, usually occurring in bedridden or wheelchair bound patients. Recent studies have shown that these ulcers can start at the skin, but also in deeper tissue layers close to bony prominences [137,138]. This pressure induced “deep tissue injury” is a major issue for wheelchair bound paraplegic patients because they are insensate to pressure-induced effects and injury is very difficult to diagnose in the absence of visible damage at the skin surface. In the studies on etiology and development of methods for prevention, biomechanical modeling is a valuable tool. The fat layer plays a very important role in these analyses and the stiffness changes described in the current paper will have a major impact on the stress and strain distributions within the different tissue layers overlying the bony prominences. This highlights the need for further research on this subject and to derive a theoretical model for the description of fat behavior. In conclusion, the time sweeps tests and the large strain experiments demonstrate that time effects and strain effects result in different material behavior. This indicates (anti-) thixotropic material behavior meaning that a constitutive model should contain parameters to describe the build-up and breakdown of material structure. When only large strains up to 0.15 are considered, a Mooney-Rivlin model should be able to capture the experimental data. The application of the Mooney-Rivlin model would demand extra parameters to include the effect of prolonged mechanical loading as well as the physiologically relevant high strains. Additionally, a power law model describing the linear viscoelastic behavior has been introduced in our previous work. This model would 88 Chapter 6 also be suitable for implementing a build-up and breakdown structure properties. We believe, however, it is better to set-up more experiments to fully understand the material behavior before continuing the building of a constitutive model. This paper shows the high complexity of the material behavior and particularly demonstrates more work is needed on this topic. The described effects should be taken into account when setting up new experiments. The follow-up experiments should clarify the effects of time and strain and the reversibility of the material. Acknowledgements We would like to thank Prof. Dan Bader for his valuable contribution to our discussions during preparation of this article. Chapter 7 General discussion 90 Chapter 7 7.1 Introductory remarks The mechanical behavior of skin is of utmost importance for clinical and cosmetic treatments. However, there is a paucity of information regarding the role of tissue mechanics in disease progression, skin-device interaction, tissue repair, and remodeling mechanisms associated with those treatments. As the skin is a challenging material composed of a layered hierarchical structure, a wide range of measurement methods for mechanical characterization of skin have been developed. Most researchers tended toward in vivo testing for obvious reasons. Non-invasive studies can then be applied on skin in its natural environment at different body sites and it is reasonably cost-effective. Although in vivo testing requires ingenious procedures and a lot of assumptions to simplify the models describing the experiment or else, numerical-experimental procedures including inverse parameters estimations, the in vivo methods are quite succesful for mechanical chararcterization of the dermis. The overall mechanical behavior of skin is often considered to be equivalent to the dermal properties. Most clinical and cosmetical applications require more detailed knowledge about individual layers at the skin surface, viable epidermis and stratum corneum, and about the deeper hypodermis. It is required to accurately measure displacements in all the layers with non-invasive methods like ultrasound, MRI, Raman spectroscopy, and Optical Coherence Tomograph. The different lengthscales, ranging from 10 μm of the stratum corneum to the cm scale for the hypodermis, and the inverse relation between penetration depth and resolution of all above mentioned techniques form a major problem. The length scales as well as the variety of stiffnesses found in the different layers also form a major difficulty for the numerical simulation tools as well as for the parameter algorithms [21]. That is why instead of investigating the mechanical behavior of human skin layers in vivo, this thesis aimed to prove that individual human skin layers can be mechanically characterized in a reliable and reproducible manner using an in vitro set-up. The layers of interest were the stratum corneum, epidermis and hypodermis, because their mechanical behavior is unknown or results in literature are inconsistent. As it is important to measure samples of consistent quality, isolation and preservation techniques for the various skin layers were analyzed. Subsequently, testing apparatus were adapted to be applicable. Because only epidermis is already a layered structure by itself, the small strain behavior was determined in an in-plane and perpendicular direction under various environmental conditions. For the hypodermis, rheological experiments were used to study the linear and non-linear behavior. In the following sections the in vitro model (Section 7.2) and the mechanical testing methods (Section 7.3) are discussed. Thereafter, the implications for clinical and cosmetic treatments (Section 7.4) and recommendations for further research (7.5) are provided. General discussion 91 7.2 In vitro model An in vitro model enables improved control of the experimental conditions and offers the potential of performing well-controlled mechanical experiments on a specific skin layer. Skin obtained from plastic surgery is preferred above cadavers, because the skin has a higher viability and skin is available from more age groups. However, the number of body sites is limited. In this thesis, skin obtained from abdominoplastic surgery was used to study the epidermis and stratum corneum. In obese people, the structure of adipose tissue has undergone changes in comparison with healthy subjects [17,139]. Therefore, a porcine model was introduced for this layer. Besides its comparable structure and function to human adipose tissue [122], the frequent availability and reproducibility of the samples was considered as very attractive. After harvesting the skin tissue, the necessary skin layer must be isolated. It has been observed that five hours after harvesting no viability could no longer observed in ex vivo mice [140]. As the time between harvesting and mechanical testing is usually too long to maintain the tissue viability and intregrity, means of preservation were needed as well. It is essential to ensure that skin preparation treatments does not have an effect on the mechanical properties. The use of ex vivo human skin in percutaneous and absorption studies is well established. Current standardized isolation and preparation protocols for skin [141] are mainly guided by cost and time effectiveness and ease of use. However, it is widely known and demonstrated that the tissue preparation influences mechanical properties [142]. In particular, the epidermal layers are known to be highly sensitive for chemical and physical changes in the environment. Therefore, available and new techniques to isolate and preserve epidermis and stratum corneum were assessed on their succesfulness regarding the maintenance of tissue integrity and viability. Furthermore, the ease of handling and the reproducibility of the protocol was considered. Much knowledge is already available from skin grafting techniques for burn wounds. However, the definition of a proper tissue condition is different from in vitro testing [77,54]. From the numerous techniques in use to isolate the epidermis, our studies showed that the number of available methods dramatically decreases when taking into account the maintenance of tissue integrity and viability. Cutting using a dermatome and enzymatic digestion with dispase fulfills both requirements and also score high on ease of handling and reproducibility. Although dispase causes the cleavage in the basement mebrane and using a dermatome not, using a dermatome is the only option for mechanical testing. In that case, the split results into a better well-defined sample geometry. As shown in Chapter 3 and 4, fragments of papillar dermis present in the epidermal samples did not influence the results for small strain behavior. As it is proven in these chapters that the mechanical behavior of stratum corneum and viable epidermis are comparable and both have a higher stiffness than de dermal layer, it can be assumed that the influence of fragments of papillar dermis in the samples can be ignored in large deformation studies as well. Regarding the isolation of the stratum corneum, the golden standard is enzymatic digestion with 0.1% trypsin. Some other techniques were analyzed as well, none showed a better performance. 92 Chapter 7 For our studies, it was preferred to store samples in HHBSS at an incubator at 37°C and 5% CO2 after separation. It should be noted that proper storage is much better achievable than during transport and the mechanical tests. Although some equipment are now being developed for controlling the environment of biological tissues, this does not hold for most apparatus yet. Usually, temperature control is built in a device but implementation of a humidity control sytem remains difficult because it might influence, for example, the sensitive load cells. The practical problems that have to be dealt with, emphasizes the importance of careful handling according to strict protocols for all skin layers. Although the dermatome was refined by the supplier, the extent of stretching the skin to use the dermatome and its intrinsic properties cause that the thickness of the epidermis was still variable. Generally, handling of the sample might induce damage, which influences the outcome of the mechanical test. The thin fragile stratum corneum easily tears during transport and cannot be placed in a set-up without using a droplet of water. Skin samples including reticular dermis curl up and twist, which makes a gently treatment challenging. Regarding the adipose tissue, every touch causes geometric deformations, which hinders the correct placement of the the sample in the set-up. 7.3 Mechanical methods In this thesis, new protocols for available reliable, accurate equipment were developed for the mechanical characterization of separate skin layers. The traditonal techniques for the in vitro mechanical characterization of skin layers are uniaxial and biaxial testing. Uniaxial tensile tests are easy to perform, cost-effective and testing equipment is a commodity in most biomechanical laboratories. Although uniaxial tensile tests do not provide sufficient information for a full characterization of the in-plane mechanical properties, it provides a means for direct comparisons between specimens, body sites, and the influence of environmental conditions for the various treatments. Biaxial testing and its interpretation are more difficult and time-consuming to perform. In addition, the equipment is more expensive and not widely available. Disadvantages of both uniaxial and biaxial testing are that it is difficult to clamp the samples without influencing the measurement, to determine the cross area due to the presence of the skin lines and to define the unloaded configuration because of the natural pre-stress in the skin. Other techniques, such as indentation and rotational shear, are able to deal with this issues and are therefore an attractive alternative for axial testing. In addition, smaller samples can be used. In order to perform these tests on our skin samples, measurement methods known for their accuracy and reliability from mechanical engineering were used: the ARES rheometer and MTS NanoIndenter XP. The major measurement problems were due to the highly non-linear viscoelastic material behavior, the low stiffness, and the sample thickness and rough surface of the epidermis and stratum corneum only. The newly developed protocols that were validated with silicone rubbers General discussion 93 resulted into a set of repoducible data for all measured skin layers. Although some problems in measuring the linear shear properties of the epidermis were encountered . In general, rheological experiments aim to characterize the viscoelastic response of soft materials, requiring relatively large homogeneous samples. To be able to obtain a homogeneous strain field as well as to increase the accuracy, an eccentric configuration was used for the upper skin layers. Temperature and humidity could be well regulated by a home-built system. The measurement chamber with controlled environment could not be closed completely, because then it would interfere with the applied shear. For that reason, the temperature and humidity sensors were placed closed to the sample to ensure a stabile environment in that area. In addition, for the upper skin layers, the required settings were close to the limitations of the apparatus. The axial resolution is 1 μm, which is close to the thickness of stratum corneum (10-20 μm). As a result, the rheometer could not be used to perform compression tests. In addition, there is some uncertaintity about the shear data for the stratum corneum, because of the thin, undulating geometry of the sample (Chapter 3). Nonetheless, the obtained resultes were reproducible, which indicates that the measurement itself is reliable and that a possible deviation in the measured response is a constant factor. Many phenomena such as the frequency-dependency and the large deformation behavior in adipose tissue could not have been measured in vivo and are also difficult to measure with other in vitro testing techniques. Since the applied protocols did not give a definitive answer on the non-linear behavior of adipose, another set of experiments aimed to describe thixotropic behavior needs to be designed. Although thixotropic studies have been extensively discussed, appropriate protocols for biological tissues are not yet available. The MTS NanoIndenter XP is more and more used to probe the mechanical response of biological materials [s]. Because of the variable probe size, indentation can be used to measure the mechanical properties from a biological material ranging from cell membranes up to the global tissue level. In addition, the system is appropriate for thin, small and heterogeneous samples. This allows testing of tissue specimens that are unsuitable for traditional mechanical testing techniques. Compared to the rheological tests on epidermis and stratum corneum, a small region of the sample is loaded with a relatively large spherical indenter to insist a more homogeneous surface and good contact during indentation. Because of the sensitivity of the load sensors, it will be challenging to regulate humidity in the future. Another related problem could be the definition of the initial sample height, because the role of adhesive forces increases. In addition, visualization of the experiment is not yet possible. Therefore, other indentation set ups as developed by Cox et al. [143] might be useful . From a mechanical point of view, the Nanoindenter XP is a very interesting technique for further research like the non-linear behavior of the upper skin layers and finally, failure behavior studies. Wu et al. [34] already developed methods to determine properties like fracture behavior from load-displacements curves of stratum corneum. 94 Chapter 7 When a direct coupling between structure and loading is essential, other methods needs to be considered as well. 7.4 Main findings 7.4.1 Small strain behavior of the epidermal layers In the present study, the stratum corneum and viable epidermis were measured in various loading directions. The variations within the studies were very small, emphasizing the reproducibility and reliability of the experimental approach. The Young‟s moduli derived from shear (in-plane) and indentation (perpendicular) studies are compared with the tensile Young‟s moduli literature in Table 7.1. For the shear experiments, the Young‟s modulus was derived from the obtained shear modulus assuming NeoHookean material behavior, such that 𝐸 = 3𝐺𝑑 . Although some authors have assessed the stiffness of the (viable) epidermis either in combination with the papillar dermis [38,144,37], this study provides the first data that are indeed obtained from epidermis only within a small strain regime. According to the highly anisotropic structure of the epidermis with the keratinocytes and corneocytes change in shape over depth, enormous differences in value exist between loading directions. The differences can be further explained by the fact that different structural components are the dominant factor during various loading types. The resistance of the keratinocytes mainly determine the mechanical response during shear, while the tensile stiffness is determined by the connections bewteen the keratinocytes, i.e. the desmosomes. As indentation is a mixture of compression, tensile and shear forces, it is less obvious which structural component is the most dominant factor. The variability in stiffness for the various loading directions emphasizes the need for an anisotropic model based on a set of experimental data in all loading directions. Another important finding is that the stiffness of the viable epidermis has a same order of magnitude in shear and indentation as the stratum corneum. This implies that the mechanical behavior of the viable epidermis cannot be ignored in the measured lengthscales. In addition, it was observed that the shear moduli are decreasing with increasing humidity, but was hardly influenced by temperature and frequency. Table 7.1: Overview of measured Young’s moduli in MPa for the stratum corneum and viable epidermis at room temperature. SHEAR INDENTATION TENSILE Eshear [MPa] Eanalytical [MPa] EFE-model [MPa] Euniaxial [MPa] Stratum corneum 25% RH 98% RH (Viable) epidermis 25% RH 0.03 0.01 0.03 2.5 n.a.* 1.1 1.2 n.a.* 1.2 40-10000 6-10 n.a * 98% RH 0.01 n.a.* n.a.* n.a.* *n.a =not available General discussion 95 7.4.2 Mechanical behavior of the subcutaneous adipose tissue For the adipose tissue, the shear modulus is about 8 kPa at 10 rad/s and 20°C and changes with temperature and frequency. The obtained modulus is in good agreement with literature [39,42]. Prolonged loading results into a dramatic stiffening of the material. This behavior is reversible with a recovery time of about 3 hours. The studies on its non-linear behavior suggest tissue structure changes with increasing strains. Up to 0.15 strain, the adipose tissue looks like to behave as a Mooney-Rivlin material. Thereafter, the stress response decreases with increasing strains and becomes stationary after 0.3 strain. Also this appeared to be reversible behavior. Although it is generally assumed in literature that adipose tissue behaves non-linear viscoelastic, these experimental data suggest thixotropic material behavior. As a consequence, this layer between the skin and muscle tissue cannot be ignored. Before numerical models can be developed, more experiments are required to fully describe the non-linear behavior of adipose tissue. 7.5 Implications for clinical and cosmetic applications The research presented in this thesis is part of larger research programmes being pursued within Philips Research and Eindhoven University of Technology (TU/e). The relevance of the work in this thesis was already shown in Chapter 1. In this section, the implications for some of the applications are discussed. In Philips Research, part of the innovation is related to consumer products that are in contact with skin, like shavers. During shaving, the skin penetrates the slits of a shaver in which the hairs are cut. To enhance shaving performance, the hair must be cut as close to the skin surface without causing irritation or other damage to the skin. The small length scale of the doming, which is the penetration of the skin in the slits, requires that the top layers are included in numerical simulations. To date, the influence of the top layer on doming has been difficult to incorporate. The underlying tissue during shaving might vary from soft tissues such as adipose tissue to bone. The material parameters of the different skin layers obtained in this study are useful to improve numerical models predicting shaver performance. Moreover, the use of hydrativing additives might affect the mechanical behavior of the top layers and thereby affect doming. At the TU/e, an ongoing research programme on the early detection and evaluation of (deep) presssure ulcers is running. Pressure ulcers are defined as areas of soft tissue breakdown that result from sustained mechanical loading of skin in shear and compression and underlying tissues. Until today, this work was mainly focused on early markers in skin [145,146] and the mechanisms associated with muscle injury [147,148,149,150]. The poor understanding of the mechanical behavior of adipose has hampered to involve this layer in the research. The reversible mechanism behavior during prolonged or severe loading of the subcutaneous fat affect the mechanisms that are initiated. In this study, the relation between mechanical behavior and tissue damage 96 Chapter 7 could not yet be made. Currently, other research groups gained interest in the role of subcutaneous adipose tissue [40,121]. Furthermore, in an in vitro study by Ohura et al [100], the purpose was to estimate the impact of external shear force and pressure on superficial skin and subcutaneous fat similtaneously. Shear force combined with a small amount of pressure is accepted as a major factor in the pathogenic mechanism of a superficial, shallow ulcer or blister. In this thesis, a method is presented to accurately measure shear forces on epidermis separately. For small strains, the epidermis is much more stiff than the dermis, which will affect the initiation of a superficial pressure ulcer. 7.6 Recommendations Some important questions remained unanswered in this thesis. The content of the thesis was focused on a reliable in vitro mechanical characterization of separate skin layers. However, to fully understand the mechanical behavior of a heteregeneous sample, it is necessary to understand how the tissue changes due to mechanical damage. The specific role of keratinocytes and desmosomes in the epidermis and the role of collagen fibers in the adipose tissue needs to be fully unraveled. In addition, real-time imaging techniques can help to solve measurement problems such as for the epidermis at high humidities. Although a variety of imaging techniques are available, factors such as the depth of imaging, resolution, field of view and the sample rate frequency are limiting the use for visualization during mechanical testing of the epidermis. For the epidermal layers, it would be interesting to track cell shape deformations by multiphoton laser scanning microscopy, allowing visualization of cellular and subcellular structures of the epidermis and upper dermis [source]. In addition, confocal imaging techniques are able to track the cell nuclei with more than 10 images per second [source]. Both techniques have the advantage that images can be obtained from intrinsic tissue properties only. This makes those techniques also appropriate for in vivo imaging and in particular, . Another imaging technique combining second-harmonic signal and 2-photon imaging is developed by Palero et al. [thesis], who demonstrated on both in vivo and ex vivo epidermal tissue from mouse that the viability of cells and the cell membranes could be measured simultaneously. In the far future, this technique is very attractive for failure studies. Before visualization of the mechanical tests on adipose tissue can be performed, it is recommended to study the fiber network surrounding the adipocytes. The relative large structures, i.e. adipocytes have a diameter up to 70 μm and are collected in lobs, limit the number of possible techniques. For instance, histological examination and confocal microscopy cannot visualize the three-dimensional structure of the collagen fiber network. Another problem is that current staining probes cannot enter thick native tissue [bron Anita]. If this can be solved, then three-dimensional techniques such as optical projection tomography can be useful. In the meantime, it does make sense to study the geometric deformations of the adipose tissue samples during mechanical behavior. In General discussion 97 particular, the response on stiffening during prolonged loading and the different phases with increasing strains have to be studied. In this thesis, only the small deformation behavior of the upper skin layers was studied. For clinical and cosmetic applications, it is essential to study the non-linear behavior of those layers as well. In principle, the experimental approaches presented in this thesis can be used to develop testing series for the non-linear region. Ultimately, experimental studies on the failure behavior is necessary. When doing so, also transport models and structural damage should be incorporated. Therefore, it is desirable to perform those studies also on in vitro human skin. Our tests proved that the non-linear behavior of adipose tissue is rather complex and cannot be captured in a constitutive model yet. Therefore, a new set of experimental data have to be collected to be able to built a constitutive model in the future. An overview of these type of tests is described by Dullaert et al. [151,152]. Mechanical tests in other loading directions should be performed as well. Compression tests are most relevant to clinical and cosmetic applications and can also be performed on a rheometer. In addition, this thesis demonstrated mechanical parameters for abdominal skin from Caucasian women in the age group of 35-55 years. Skin with striae, cellulite, UV damage or excessively hairy skin was excluded from the study. Other studies should include other skin types, other body sites with a high density of hairs or UV exposure, ageing effects, and so on. Ultimately, a full-thickness constitutive model consisting of individual skin layers may be developed not only to study damage development, but also to serve as a model for investigating new prevention and treatment strategies. For applications such as decubitus, it is advised to incorporate transport models, which offers the potential to asses the correlation between tissue damage and biological markers. 7.7 General conclusion This thesis presents methods to determine mechanical properties of individual skin layers in vitro. The two main findings are: 1) the stratum corneum and viable epidermis behave highly anisotropic in the small strain behavior and the stiffness of the viable epidermis is equivalent to that of the stratum corneum in each loading direction, and 2) the hypodermis initially shows typical small strain behavior for soft tissues, but seems to behave thixotropically during prolonged deformation and for larger strains. These two main findings highlight the importance of mechanical characterization of individual skin layers as well as the need for anisotropic models involving separate skin layers in numerical simulations. The used experimental methods represent valuable tools for studying the mechanical properties in relation to disease and treatments in future. 98 Chapter 7 Samenvatting De mechanische eigenschappen van de menselijke huid zijn van belang voor vele klinische en cosmetische toepassingen. Vaak wordt de huid beschouwd als één geheel, maar inmiddels is gebleken dat het voor diverse toepassingen van belang is het mechanische gedrag van de afzonderlijke huidlagen te begrijpen. Voorbeelden hiervan zijn: het toedienen van medicijnen door de huid, de interactie tussen de huid en een (scheer)apparaat en de preventie en behandeling van doorligwonden. Totnutoe is veel onderzoek naar de mechanische eigenschappen van de huid uitgevoerd in an in vivo situatie waarbij aangenomen dat de middelste huidlaag met zijn vezelstructuur representatief is voor de huid in zijn geheel. Het doel van dit promotieonderzoek was om de mechanische eigenschappen van de afzonderlijke huidlagen te karakteriseren. Hierbij is specifiek gericht op die huidlagen waarvan nog nauwelijks literatuur beschikbaar is of de resultaten in de literatuur inconsistent zijn. Allereerst is er onderzocht wat de beste methoden zijn om de verschillende huidlagen van elkaar te scheiden en levensvatbaar te houden in an in vitro omgeving. Aandachtspunten hierbij waren het effect van een methode op de weefselstructuur en de levensvatbaarheid alsook de betrouwbaarheid, duur en de mate van uitvoerbaarheid van een methode. Hieruit kon geconcludeerd worden dat voor dit onderzoek de epidermis het beste geisoleerd kan worden met een dermatoom. Vervolgens is de epidermis bewaard in een medium, HHBSS, of door in te vriezen volgens een specifieke protocol. De stratum corneum kan van de epidermis geisoleerd te worden door gebruik te maken van het enzym trypsin om vervolgens bewaard te worden in een saline buffer, PBS, of in gedroogde vorm. Vervolgens zijn er verschillende methoden ontwikkeld om de mechanische reactie van de afzonder huidlagen te kunnen meten. Voor de bovenste huidlagen, de opperhuid en hoornlaag, zijn in vitro meetopstellingen gebouwd om de respons op kleine rekken te kunnen meten. In het dagelijks leven, worden grote rekken in principe opgevangen door het ontvouwen van het huidoppervlak en dus alleen bij niet-fysiologische omstandigheden, zoals een naald door de huid prikken, zal de epidermis grote rekken ondergaan. Omdat schuif en druk sterk aan elkaar gerelateerd zijn en het bekend is dat de opperhuid een inhomogene gelaagde structuur heeft, is er gekozen voor het opleggen van zowel een schuif- als indentatiebelasting. Voor beide soorten belasting is aangetoond dat 100 Samenvatting er geen significant verschil is tussen de mechanische eigenschappen van de epidermis en stratum corneum. Verder bleken deze huidlagen bij een schuifrek wel gevoelig voor vochtigheid maar niet voor temperatuuur. Als de kracht loodrecht op de huid staat, gedraagt de opperhuid zich veel stijver dan bij het opleggen van een schuifrek. De uitkomsten van deze experimenten tonen aan dat het essentieel is om het anisotrope gedrag van deze afzonderlijke huidlagen mee te nemen in numerieke huidmodellen. De onderhuidse vetlaag is belast met kleine en grote schuifrekken gedurende korte en lange termijn. De frequentie en temperatuur afhankelijkheid van de mechanische parameters zijn gemeten bij kleine rekken. Het is gebleken dat al bij zeer kleine rekken de onderhuidse vetlaag ernstig gaat vervormen na langdurige belasting, maar dat na een rustperiode het gedrag reversibel is. Dit duidt erop dat er veranderingen in de weefselstructuur optreden door mechanische belasting maar zonder blijvende schade. Ook het opleggen van grote schuifrekken resulteerde in veranderingen in de weefselstructuur die reversibel bleken. Tot zekere schuifrekken is het gedrag van onderhuidsvet vergelijkbaar met andere zachte lichaamsweefsels. Bij zeer hoge schuifrekken wordt het materiaalgedrag complexer. Om dit goed te kunnen begrijpen, zijn er eerst meer experimenten nodig voordat er numerieke modellen gebouwd kunnen worden die ook deze grote schuifrekken kunnen beschrijven. Een goede basis voor een numeriek model zou een Mooney-Rivlin of powerlaw model kunnen zijn. In dit proefschrift zijn mechanische eigenschappen van individuele huidlagen bepaald in een in vitro omgeving met behulp van nauwkeurige apparatuur, resulterend in reproduceerbare resultaten. Het wordt aanbevolen om in de toekomst de relatie tussen de deformatie in het weefsel en het mechanisch gedrag te bestuderen met behulp van visualizatietechnieken. Daarnaast zal het onderzoek uitgebreid moeten worden met studies naar het faalgedrag van de individuele huidlagen in relatie tot klinische en cosmetische applications. Dankwoord Graag wil ik iedereen bedanken die direct of indirect een bijdrage heeft geleverd aan de totstandkoming van dit proefschrift. Een aantal mensen wil ik specifiek bedanken. Allereerst wil ik Frank en Paco bedanken voor het mogelijk maken van mijn project binnen deze bijzondere constructie tussen Philips en TU/e. Door de samenwerking heb ik kunnen profiteren van de faciliteiten van beide zijdes alsook van de kennis over de huid als van de (bio)mechanica. Cees, bedankt voor het vertrouwen en je positieve relativerende kijk op zaken. Zonder jou en Sigi had ik de stap om te gaan promoveren nooit genomen. Gerrit, bedankt voor het kijkje in de wereld van de polymeren en rheologie. Hoewel ik je kunstzinnige hierogliefen tegenwoordig lees alsof het geschreven is in Times New Roman is, zal ik ze toch gaan missen! Paul, ik vind je enthousiasme, vertrouwen, en kritische blik altijd erg bijzonder. Bedankt dat je altijd voor me klaar stond! Dear Dan, I really appreciate your contribution to my thesis. Daarnaast zijn er nog een aantal mensen die me op praktisch vlak vooruit hebben geholpen. Hoewel al een poosje terug, wil ik Matej en Evelyne bedanken voor de kennismaking met het meten aan zachte weefsels aan de rheometer. Ik wist toen nog niet dat het rheohok mijn huiskamer zou gaan worden. Henk en ook de mannen van de TU werkplaats, bedankt voor de mooie verzameling rheometer hulpstukken! Lambert, we hadden samen een voorbeeldig MaTe-project met jouw W en mijn BMT achtergrond, en dan ook nog experimenteel en numeriek. Jan, ik ben zeer blij dat mijn statistiekproblemen voor jouw een wetenschappelijke uitdaging waren. Sjoerd, zullen we nog een keer een speklapje opeten, terwijl je de kurkboor scherp maakt? Sarita, bedankt voor al het snij- en kleurwerk dat je voor me gedaan hebt. Henny, jouw tekeningen hebben dit boekje aanzienlijk opgefleurd. Ik wil de stagaires Francois, Roman Ditmar en Suzanne Stolk en verscheidene derdejaars projectgroepjes bedanken voor hun bijdrage in het onderzoek. Lisette, Debbie, Roel en Susanne, fijn dat er ook andere mensen met ex vivo huid bezig waren. Anke, jij bent ook zeker een bedankje waard. In een samenwerkingsverband tussen Philips en TU/e heb ik veel dubbel mogen beleven. Het is erg bijzonder om te werken in twee groepen met zoveel collegae. Ik zou mijn kamergenootjes bij Philips alsook op de TU/e specifiek willen bedanken voor hun gezelschap. Rachel, goed bezig! I‟m glad that someone invented Facebook to keep sharing our daily complaints and gossips! Anke en Maria, ik blijf het leuk vinden om af 102 Dankwoord en toe het vijfde wiel aan de wagen te zijn en hoop dan ook dat er nog veel etentjes komen! Ik wil het personeel van de afdelingen plastische chirurgie en de operatiekamers in het Catharina Ziekenhuis in Eindhoven bedanken voor alle emmertjes met huid. In het bijzonder de plastisch chirurgen Van Rappard en Hoogbergen die deze samenwerking mogelijk hebben gemaakt alsook Marjolein (en je directe collega‟s) en de OK-receptie voor alle telefoongesprekken. Lieve OLT en andere scoutingvriendjes, het is erg relativerend om een potje te koken en een biertje te drinken in het bos, bij een kampvuur, in de disco of in de kroeg. Na al die jaren en kampen blijft het gezellig en voor mij erg waardevol! Vrouwenweekendjes (en de autorit heen en terug, Margo!) ben ik ook gaan waarderen. Daarnaast is het erg leuk om in de wachttijd van een experiment over de scoutingorganisatie na te denken: regiegroep, grote kampen, Georgie, enz., enz. Peter, mutsen en onderbroeken staan garant voor leuke herinneringen. Ik ben benieuwd welke kledingstukken we de komende jaren er nog bij weten te verzamelen. Frank, Pe, Xander en Elizabeth, Nicole, en alle anderen bedankt voor jullie interesse in mijn onderzoek. Lieve Iksiks, zonder Betty Boo en mijn roze kledingset was mijn promotietijd toch een stuk minder vrolijk geweest! Nicole en Jannet, ik heb weer tijd voor onze etentjes en bezoekjes aan ons wereldwijde vriendennetwerk (sorry!). Lieve Rianne, ik heb weer zeeen van tijd voor onzinnige projectjes. Ook mijn wandelstokken en bergschoenen staan te popelen (wordt het een graad 4?). Lieve papa en mama, dankjullie wel voor jullie geduld. Het komt wel goed met me. Gerrie, Dick en Sebas, het is erg ontspannend om met zo‟n gezellige schoonfamilie op stap te zijn! Lieve Martijn, altijd komt toch alles goed? Maar eerlijk is eerlijk, zonder jouw luisterend oor (ergens in een auto), je releativerende woorden en onvoorwaardelijke steun had ik het nooit gered. Ga je mee naar Nice? Marion Geerligs, Eindhoven, november 2009. Curriculum Vitae Marion Geerligs is geboren op 21 juni 1979 in Hoogezand-Sappemeer. In 1998 behaalde zij haar Gymnasium diploma aan het CSG Vincent van Gogh in Assen. Aansluitend studeerde zij een jaar Bewegingswetenschappen aan de Vrije Universiteit Amsterdam. Na een jaar besloot zij over te stappen naar de studie Biomedische Technologie aan de Technische Universiteit Eindhoven. Als onderdeel van deze studie liep zij stage in het St. Mary Hospital in Mumias (Kenia), waar zij onderzoek deed naar de preventie van doorligwonden bij aan bedgebonden patienten. Haar afstudeerwerk richtte zich op het ontwerpen van een testobject voor ge-automatiseerd bloed prikken waarin de mechanische en ultrasoundeigenschappen van de huid, vet, vaatwand en bloed werden nagebootst. Dit onderzoek werd uitgevoerd binnen de groep Care & Health Applications van Philips Research. Vanwege haar interesse in het onderzoek naar de biomechanica van zachte weefsels besloot zij in 2005 verder te gaan met een promotieonderzoek bij dezelfde groep in een samenwerkingsverband met de Technische Universiteit Eindhoven. References [1] Berson, M., Vabre, V., Karlsson, B., Gregoire, J. M., Yvon, C., Patat, F., Auriol, F., Vaillant, L., Diridollou, S., Black, D., and Gall, Y., (1998). An in vivo method for measuring the mechanical properties of the skin using ultrasound. Ultrasound in Medicine and Biology, 215-224, 24(2), 215-224. [2] Plewig, G. and Marples, R. R., (1970). Regional differences of cell sizes in the human stratum corneum. I. J.Invest Dermatol., 13-18, 54(1), 13-18. [3] Holbrook, K. A. and Odland, G. F., (1974). Regional differences in the thickness (cell layers) of the human stratum corneum: an ultrastructural analysis. J.Invest Dermatol., 415-422, 62(4), 415-422. [4] Brody, I., (1977). Ultrastructure of the stratum corneum. Int.J.Dermatol., 245-256, 16(4), 245-256. [5] Agache, P. and Humbert, P. (2004). Measuring the skin. Springer, [6] Plewig, G., (1970). Regional differences of cell sizes in the human stratum corneum. II. Effects of sex and age. J.Invest Dermatol., 19-23, 54(1), 19-23. [7] Rawlings, A. V., Scott, L. R., Harding, C. R., and Bowser, P. A., (1994). Stratum corneum moisturization at the molecular level. Journal of Investigative Dermatology, 731-740, 103(5), 731-740. [8] Agache, P., Boyer, J. P., and Laurent, R., (1973). Biomechanical properties and microscopic morphology of human stratum corneum incubated on a wet pad in vitro. Archives of Dermatological Research, 271-283, 246(3), 271-283. [9] Marks, R., (2004). The stratum corneum barrier: The final frontier. Journal of Nutrition, 134(8 SUPPL.), [10] Elias, P. M. and Feingold, K. R. (2005). Skin Barrier. Informa Healthcare, References 105 [11] Blank, I. H., Moloney, J., III, Emslie, A. G., Simon, I., and Apt, C., (1984). The diffusion of water across the stratum corneum as a function of its water content. J.Invest Dermatol., 188-194, 82(2), 188-194. [12] Sandby-M°ller, J., Wulf, H. C., and Poulsen, T., (2003). Epidermal Thickness at Different Body Sites: Relationship to Age, Gender, Pigmentation, Blood Content, Skin Type and Smoking Habits. Acta Dermato-Venereologica, 410-413, 83(6), 410413. [13] Silver, F. H., Siperko, L. M., and Seehra, G. P., (2003). Mechanobiology of force transduction in dermal tissue. Skin Research and Technology, 3-23, 9(1), 3-23. [14] Montagna, W., Kligman, A. M., and Carlisle, K. S. (1992). Atlas of Normal Human Skin. Springer Verlag, New York. [15] Odland, G., (1991). Structure of skin. In: Goldsmith, L. A., Physiology, biochemistry, and molecular biology of the skin. Oxford University Press, Oxford. [16] Briggaman, R. A. and Wheeler, C. E., Jr., (1975). The epidermal-dermal junction. J.Invest Dermatol., 71-84, 65(1), 71-84. [17] Avram, A. S., Avram, M. M., and James, W. D., (2005). Subcutaneous fat in normal and diseased states: 2. Anatomy and physiology of white and brown adipose tissue. Journal of the American Academy of Dermatology, 671-683, 53(4), 671683. [18] Ann L.Albright & Judith S.Stern, Dept of Nutrition and Internal Medicine Unicersity of California at Davis,(2005). adipose tissue. [19] Shen, W., Wang, Z, Punyanita, M, Lei, J., Sinav, A, Kral, J. G., Imielinska, C., Ross, R., and Heymsfield, S. B., (2005). Adipose tissue quantification by imaging methods. Obesity research, 5-11, 11(1), 5-11. [20] Elsner, P., Berardesca, E., Wilhelm, K. P., and Maibach, H. I. (2002). Bioengineering of the skin; Skin Biomechanics. CRC Press, [21] Hendriks, F. M. (2005). Mechanical Behaviour of Human Epidermal and Dermal Layers. Ph.D. thesis, Technische Universiteit Eindhoven. [22] Wildnauer, R. H., Bothwell, J. W., and Douglass, A. B., (1971). Stratum corneum biomechanical properties. I. Influence of relative humidity on normal and extracted human stratum corneum. Journal of Investigative Dermatology, 72-78, 56(1), 7278. 106 References [23] Park, A. C. and Baddiel, C. B., (1972). Rheology of stratum corneum-I: A molecular interpretation of the stress-strain curve. Jourbal of Society Cosmetical Chemistry, 3-12, 233-12. [24] Papir, Y. S., Hsu, K. H., and Wildnauer, R. H., (1975). The mechanical properties of stratum corneum. I. The effect of water and ambient temperature on the tensile properties of newborn rat stratum corneum. Biochimica et Biophysica Acta, 170180, 399(1), 170-180. [25] Wolfram, M. A., Wolejsza, N. F., and Laden, K., (1972). Biomechanical properties of delipidized stratum corneum. J.Invest Dermatol., 421-426, 59(6), 421-426. [26] Van Duzee, B. F., (1978). The influence of water content, chemical treatment and temperature on the rheological properties of stratum corneum. Journal of Investigative Dermatology, 140-144, 71(2), 140-144. [27] de Rigal, J. and Leveque, J. L., (1985). In vivo measurement of the stratum corneum elasticity. Bioengineering and the Skin, 13-23, 1(1), 13-23. [28] Agache, P. G., Monneur, C., Leveque, J. L., and de Rigal, J., (1980). Mechanical properties and Young's modulus of human skin in vivo. Archives of Dermatological Research, 221-232, 269(3), 221-232. [29] Leveque, J. L., de Rigal, J., Agache, P. G., and Monneur, C., (1980). Influence of ageing on the in vivo extensibility of human skin at a low stress. Archives of Dermatological Research, 127-135, 269(2), 127-135. [30] Pierard, G. E., Nikkels-Tassoudji, N., and Pierard-Franchimont, C., (1995). Influence of the test area on the mechanical properties of skin. Dermatology, 9-15, 191(1), 9-15. [31] Yonghui, Y. and Verma, R., (2003). Mechanical properties of stratum corneum studied by nano-indentation. Spatially Resolved Characterization of Local Phenomena in Materials and Nanostructures.Symposium (Mater.Res.Soc.Symposium Proceedings Vol.738), 265-272, 265-272. [32] Gardner, T. N. and Briggs, G. A. D., (2001). Biomechanical measurements in microscopically thin stratum corneum using acoustics. Skin Research and Technology, 254-261, 7(4), 254-261. [33] Graves, C. J. and Edwards, C., (2002). Hardware and Measuring Principles: The Microindentometer. In: Elsner, P., Berardesca, E., Wilhelm, K. P., and Maibach, H. I., Bioengineering of the skin: skin biomechanics. CRC Press LLC, Boca Raton. References 107 [34] Wu, K. S., van Osdol, W. W., and Dauskardt, R. H., (8-8-2005). Mechanical properties of human stratum corneum: Effects of temperature, hydration, and chemical treatment. Biomaterials, [35] Koutroupi, K. S. and Barbenel, J. C., (1990). Mechanical and failure behaviour of the stratum corneum. J.Biomech., 281-287, 23(3), 281-287. [36] Middleton, J. D., (1969). The effect of temperature on extensibility of isolated corneum and its relation to skin chapping. Br.J.Dermatol., 717-721, 81(10), 717721. [37] Kendall, M. A., Chong, Y. F., and Cock, A., (2007). The mechanical properties of the skin epidermis in relation to targeted gene and drug delivery. Biomaterials, 4968-4977, 28(33), 4968-4977. [38] Hendriks, F. M., Oomens, C. W. J., Bader, D. L., Baaijens, F. P. T., and Brokken, D., (2006). The relative contributions of different skin layers to the mechanical behavior of human skin in vivo using suction experiments. Medical Engineering and Physics, 259-266, 28(3), 259-266. [39] Patel, P. N., Smith, C. K., and Patrick, C. W., (2005). Rheological and recovery properties of poly(ethylene glycol) diacrylate hydrogels and human adipose tissue. Journal of Biomedical Materials Research Part A, 313-319, 73A(3), 313-319. [40] Gefen, A. and Haberman, E., (2007). Viscoelastic properties of ovine adipose tissue covering the gluteus muscles. Journal of Biomechanical Engineering-Transactions of the Asme, 924-930, 129924-930. [41] Samani, A. and Plewes, D., (21-9-2004). A method to measure the hyperelastic parameters of ex vivo breast tissue samples. Phys.Med.Biol., 4395-4405, 49(18), 4395-4405. [42] Samani, A., Zubovits, J., and Plewes, D., (21-3-2007). Elastic moduli of normal and pathological human breast tissues: an inversion-technique-based investigation of 169 samples. Phys.Med.Biol., 1565-1576, 52(6), 1565-1576. [43] Samani, A., Zubovits, J., and Plewes, D., (2007). Elastic moduli of normal and pathological human breast tissues: an inversion-technique-based investigation of 169 samples. Physics in Medicine and Biology, 1565-1576, 52(6), 1565-1576. [44] Van Houten, E. E., Doyley, M. M., Kennedy, F. E., Weaver, J. B., and Paulsen, K. D., (2003). Initial in vivo experience with steady-state subzone-based MR elastography of the human breast. J.Magn Reson.Imaging, 72-85, 17(1), 72-85. 108 References [45] Skerrow, D. and Skerrow, S. J., (1985). A Survey of Methods for the Isolation and Fractionation of Epidermal Tissue and Cells. In: D.Skerrow and C.J.Skerrow, Methods in Skin Research. John Wiley & Sons Ltd., New York. [46] Messager, S., Hann, A. C., Goddard, P. A., Dettmar, P. W., and Maillard, J. Y., (2003). Assessment of skin viability: is it necessary to use different methodologies? Skin Research and Technology, 321-330, 9(4), 321-330. [47] Basaran, O., Ozdemir, H., Kut, A., Sahin, F. I., Deniz, M., Sakallioglu, E. A., and Haberal, M. A., (2006). Effects of different preservation solutions on skin graft epidermal cell viability and graft performance in a rat model. Burns, 423-429, 32(4), 423-429. [48] Collier, S. W., Sheikh, N. M., Sakr, A., Lichtin, J. L., Stewart, R. F., and Bronaugh, R. L., (1989). Maintenance of skin viability during in vitro percutaneous absorption/metabolism studies. Toxicol.Appl.Pharmacol., 522-533, 99(3), 522-533. [49] D.Skerrow and C.J.Skerrow, (1985). A Survey of Methods for the Isolation and Fractionation of Epidermal Tissue and Cells. In: D.Skerrow and C.J.Skerrow, Methods in Skin Research. John Wiley & Sons Ltd., New York. [50] Willsteed, E. M., Bhogal, B. S., Das, A., Bekir, S. S., Wojnarowska, F., Black, M. M., and McKee, P. H., (1991). An ultrastructural comparison of dermo-epidermal separation techniques. J.Cutan.Pathol., 8-12, 18(1), 8-12. [51] Steinstrasser, I. and Merkle, H. P., (1995). Dermal metabolism of topically applied drugs: pathways and models reconsidered. Pharm.Acta Helv., 3-24, 70(1), 3-24. [52] Ogura, R., Knox, J. M., and GRIFFIN, A. C., (1960). Separation of epidermis for the study of epidermal sulfhydryl. J.Invest Dermatol., 239-243, 35239-243. [53] VAN SCOTT, E. J., (1952). Mechanical separation of the epidermis from the corium. J.Invest Dermatol., 377-379, 18(5), 377-379. [54] Bravo, D., Rigley, T. H., Gibran, N., Strong, D. M., and Newman-Gage, H., (2000). Effect of storage and preservation methods on viability in transplantable human skin allografts. Burns, 367-378, 26(4), 367-378. [55] Kiistala, U., (1968). Suction Blister Device for Separation of Viable Epidermis from Dermis. Journal of Investigative Dermatology, 129-&, 50(2), 129-&. [56] Beerens, E. G., Slot, J. W., and van der Leun, J. C., (1975). Rapid regeneration of the dermal-epidermal junction after partial separation by vacuum: an electron microscopic study. J.Invest Dermatol., 513-521, 65(6), 513-521. References 109 [57] COWDRY, E. V., CARRUTHERS, C., and SUNTZEFF, V., (1948). Influence of age on the copper and zinc contest in the epidermis of mice undergoing carcinogenesis with methylcholanthrene and a note on the role of calcium. J.Natl.Cancer Inst., 209-213, 8(5-6), 209-213. [58] Baumberger, J. P., Suntzeff, V., and Cowdry, E. V., (1965). Methods for the Separation of epidermis from Dermis and Some Physiologic and Chemical Properties of Isolated Epidermis. Journal of the National Cancer Institute, 413-423, 413-423. [59] Dimond, R. L., Erickson, K. L., and Wuepper, K. D., (1976). The role of divalent cations in epidermolysis. Br.J.Dermatol., 25-34, 95(1), 25-34. [60] Scaletta, L. J., Occhino, J. C., MacCallum, D. K., and Lillie, J. H., (1978). Isolation and immunologic identification of basement membrane zone antigens from human skin. Lab Invest, 1-9, 39(1), 1-9. [61] Woodley, D., Sauder, D., Talley, M. J., Silver, M., Grotendorst, G., and Qwarnstrom, E., (1983). Localization of basement membrane components after dermal-epidermal junction separation. J.Invest Dermatol., 149-153, 81(2), 149-153. [62] Baden, H. P. and Gifford, A. M., (1970). Isometric contraction of epidermis and stratum corneum with heating. J.Invest Dermatol., 298-303, 54(4), 298-303. [63] Wester, R. C., Christoffel, J., Hartway, T., Poblete, N., Maibach, H. I., and Forsell, J., (1998). Human cadaver skin viability for in vitro percutaneous absorption: storage and detrimental effects of heat-separation and freezing. Pharm.Res., 82-84, 15(1), 82-84. [64] Briggaman, R. A., (1982). Biochemical composition of the epidermal-dermal junction and other basement membrane. J.Invest Dermatol., 1-6, 78(1), 1-6. [65] Walzer, C., Benathan, M., and Frenk, E., (1989). Thermolysin treatment: a new method for dermo-epidermal separation. J.Invest Dermatol., 78-81, 92(1), 78-81. [66] Liu, S. C. and Karasek, M., (1978). Isolation and growth of adult human epidermal keratinocytes in cell culture. J.Invest Dermatol., 157-162, 71(2), 157-162. [67] Stenn, K. S., Link, R., Moellmann, G., Madri, J., and Kuklinska, E., (1989). Dispase, a neutral protease from Bacillus polymyxa, is a powerful fibronectinase and type IV collagenase. J.Invest Dermatol., 287-290, 93(2), 287-290. [68] Kitano, Y. and Okada, N., (1983). Separation of the epidermal sheet by dispase. Br.J.Dermatol., 555-560, 108(5), 555-560. 110 References [69] Geggel, H. S. and Gipson, I. K., (1985). Removal of viable sheets of conjunctival epithelium with dispase II. Invest Ophthalmol.Vis.Sci., 15-22, 26(1), 15-22. [70] Sanchez, M. A., Diaz, N. L., and Tapia, F. J., (2002). Microwave irradiation for rapid epidermis-dermis separation and improved epidermal cell immunodetection. Biotech.Histochem., 183-187, 77(4), 183-187. [71] Kholodnykh, A. I., Petrova, I. Y., Larin, K. V., Motamedi, M., and Esenaliev, R. O., (1-6-2003). Precision of measurement of tissue optical properties with optical coherence tomography. Appl.Opt., 3027-3037, 42(16), 3027-3037. [72] Ferguson, J., (1977). A method to facilitate the isolation and handling of stratum corneum. British Journal of Dermatology, 21-23, 96(1), 21-23. [73] Humphries, W. T. and Wildnauer, R. H., (1971). Thermomechanical analysis of stratum corneum. I. Technique. J.Invest Dermatol., 32-37, 57(1), 32-37. [74] Humphries, W. T. and Wildnauer, R. H., (1971). Thermomechanical analysis of stratum corneum. I. Technique. J.Invest Dermatol., 32-37, 57(1), 32-37. [75] Nicollier, M., Agache, P., Kienzler, J. L., Laurent, R., Gibey, R., Cardot, N., and Henry, J. C., (1980). Action of trypsin on human plantar stratum corneum. An ultrastructural study. Arch.Dermatol.Res., 53-64, 268(1), 53-64. [76] Morejohn, L. C. and Pratley, J. N., (18-5-1979). Differential effects of trypsin on the epidermis of Rana catesbeiana. Observations on differentiating junctions and cytoskeletons. Cell Tissue Res., 349-362, 198(2), 349-362. [77] Kearney, J. N., (2005). Guidelines on processing and clinical use of skin allografts. Clin.Dermatol., 357-364, 23(4), 357-364. [78] Zieger, M. A. J., Tredget, E. E., and McGann, L. E., (1996). Mechanisms of cryoinjury and cryoprotection in split-thickness skin. Cryobiology, 376-389, 33(3), 376-389. [79] Ben Bassat, H., (2005). Performance and safety of skin allografts. Clin.Dermatol., 365-375, 23(4), 365-375. [80] Meyer, W., Zschemisch, N. H., and Godynicki, S., (2003). The porcine ear skin as a model system for the human integument: influence of storage conditions on basic features of epidermis structure and function--a histological and histochemical study. Pol.J.Vet.Sci., 17-28, 6(1), 17-28. References 111 [81] De, A., Mathur, M., and Gore, M. A., (2008). Viability of cadaver skin grafts stored in skin bank at two different temperatures. Indian J.Med.Res., 769-771, 128(6), 769-771. [82] Castagnoli, C., Alotto, D., Cambieri, I., Casimiri, R., Aluffi, M., Stella, M., Alasia, S. T., and Magliacani, G., (2003). Evaluation of donor skin viability: fresh and cryopreserved skin using tetrazolioum salt assay. Burns, 759-767, 29(8), 759-767. [83] Han, B. and Bischof, J. C., (2004). Engineering Challenges in Tissue Preservation. Cell Preservation Technology, 91-112, 2(2), 91-112. [84] Lawrence, J. C., (1972). Storage and skin metabolism. Br.J.Plast.Surg., 440-453, 25(4), 440-453. [85] Foutz, T. L., Stone, E. A., and Abrams, Jr, (1992). Effects of freezing on mechanical properties of rat skin. American journal of veterinary research, 788-792, 53(5), 788-792. [86] Bouwstra, J. A.,(2009). [87] Bouwstra, J. A., de Graaff, A., Gooris, G. S., Nijsse, J., Wiechers, J. W., and van Aelst, A. C., (2003). Water distribution and related morphology in human stratum corneum at different hydration levels. Journal of Investigative Dermatology, 750758, 120(5), 750-758. [88] Chistolini, P., De Angelis, G., De Luca, M., Pellegrini, G., and Ruspantini, I., (1999). Analysis of the mechanical properties of in vitro reconstructed epidermis: preliminary results. Medical & Biological Engineering & Computing, 670-672, 37(5), 670-672. [89] Rochefort, A., Druot, P., Leduc, M., Vassalet, R., and Agache, P., (1986). A New Technique for the Evaluation of Cosmetics Effect on Mechanical-Properties of Stratum-Corneum and Epidermis Invitro. International Journal of Cosmetic Science, 27-36, 8(1), 27-36. [90] Geerligs, M,(2007). Mechanical behaviour of human skin layers: stratum corneum, living epidermis and hypodermis. A literature review. PR-TN 2006/00450( [91] van Turnhout, M., Peters, G., Stekelenburg, A., and Oomens, C., (2005). Passive transverse mechanical properties as a function of temperature of rat skeletal muscle in vitro. Biorheology, 193-207, 42(3), 193-207. [92] Hrapko, M., van Dommelen, J. A., Peters, G. W., and Wismans, J. S., (2006). The mechanical behaviour of brain tissue: large strain response and constitutive modelling. Biorheology, 623-636, 43(5), 623-636. 112 References [93] van Dam, E. A., Dams, S. D., Peters, G. W., Rutten, M. C., Schurink, G. W., Buth, J., and van de Vosse, F. N., (2006). Determination of linear viscoelastic behavior of abdominal aortic aneurysm thrombus. Biorheology, 695-707, 43(6), 695-707. [94] Holt, B., Tripathi, A., and Morgan, J., (28-8-2008). Viscoelastic response of human skin to low magnitude physiologically relevant shear. J.Biomech., 2689-2695, 41(12), 2689-2695. [95] Gennisson, J. L., Baldeweck, T., Tanter, M., Catheline, S., Fink, M., Sandrin, L., Cornillon, C., and Querleux, B., (2004). Assessment of elastic parameters of human skin using dynamic elastography. Ieee Transactions on Ultrasonics Ferroelectrics and Frequency Control, 980-989, 51(8), 980-989. [96] Park, A. C. and Baddiel, C. B., (1972). Rheology of Stratum Corneum .2. Physicochemical Investigation of Factors Influencing Water-Content of Corneum. Journal of the Society of Cosmetic Chemists, 13-&, 23(1), 13-&. [97] Wu, K. S., Li, J., Ananthapadmanabhan, K. P., and Dauskardt, R. H., (2007). Timedependant intercellular delamination of human stratum corneum. Journal of Materials Science, 8986-8994, 42(21), 8986-8994. [98] vanHal, D. A., Jeremiasse, E., Junginger, H. E., Spies, F., and Bouwstra, J. A., (1996). Structure of fully hydrated human stratum corneum: A freeze fracture electron microscopy study. Journal of Investigative Dermatology, 89-95, 106(1), 89-95. [99] Warner, R. R., Stone, K. J., and Boissy, Y. L., (2003). Hydration disrupts human stratum corneum ultrastructure. Journal of Investigative Dermatology, 275-284, 120(2), 275-284. [100] Ohura, T., Takahashi, M., and Ohura, N., Jr., (2008). Influence of external forces (pressure and shear force) on superficial layer and subcutis of porcine skin and effects of dressing materials: are dressing materials beneficial for reducing pressure and shear force in tissues? Wound.Repair Regen., 102-107, 16(1), 102-107. [101] Pailler-Mattei, C., Pavan, S., Vargiolu, R., Pirot, F., Falson, F., and Zahouani, H., (2007). Contribution of stratum corneum in determining bio-tribological properties of the human skin. Wear, 1038-1043, 263(7-12 SPEC. ISS.), 1038-1043. [102] Hendley, A., Marks, R., and Payne, P. A., (1982). Measurement of forces for point indentation of the stratum corneum in vivo: The influences of age, sex, site delipidisation and hydration. Bioeng Skin, 234-240, 3234-240. References 113 [103] Pailler-Mattei, C. and Zahouani, H., (2004). Study of adhesion forces and mechanical properties of human skin in vivo. Journal of Adhesion Science and Technology, 1739-1758, 18(15-16), 1739-1758. [104] Potter, A., Luengo, G., Baltenneck, C., Pavan, S., and Loubet, JL,(1-7-2007). Measuring mechanical properties of stratum corneum and isolated corneocytes at sub-micron length scale. [105] Plewig, G. and Marples, R. R., (1970). Regional differences of cell sizes in the human stratum corneum. I. J.Invest Dermatol., 13-18, 54(1), 13-18. [106] Plewig, G., (1970). Regional differences of cell sizes in the human stratum corneum. II. Effects of sex and age. J.Invest Dermatol., 19-23, 54(1), 19-23. [107] Yuan, Y. and Verma, R., (2006). Measuring microelastic properties of stratum corneum. Colloids and Surfaces B: Biointerfaces, 6-12, 48(1), 6-12. [108] Oliver, W. C. and Pharr, G. M., (1992). An Improved Technique for Determining Hardness and Elastic-Modulus Using Load and Displacement Sensing Indentation Experiments. Journal of Materials Research, 1564-1583, 7(6), 1564-1583. [109] Pelletier, C. G. N., Den Toonder, J. M. J., Govaert, L. E., Hakiri, N., and Sakai, M., (2008). Quantitative assessment and prediction of contact area development during spherical tip indentation of glassy polymers. Philosophical Magazine, 12911306, 88(9), 1291-1306. [110] Ebenstein, D. M. and Pruitt, L. A., (2006). Nanoindentation of biological materials. Nano Today, 26-33, 1(3), 26-33. [111] Jachowicz, J., McMullen, R., and Prettypaul, D., (2007). Indentometric analysis of in vivo skin and comparison with artificial skin models. Skin Research and Technology, 299-309, 13(3), 299-309. [112] Boyer, G., LaquiFze, L., Le Bot, A., LaquiFze, S., and Zahouani, H., (2009). Dynamic indentation on human skin in vivo: ageing effects. Skin research and technology : official journal of International Society for Bioengineering and the Skin (ISBS) [and] International Society for Digital Imaging of Skin (ISDIS) [and] International Society for Skin Imaging (ISSI), 55-67, 15(1), 55-67. [113] Iatridis, J. C., Wu, J., Yandow, J. A., and Langevin, H. M., (2003). Subcutaneous tissue mechanical behavior is linear and viscoelastic under uniaxial tension. Connect.Tissue Res., 208-217, 44(5), 208-217. [114] Haberman, E. and Gefen, A.,(2007). Viscoelastic properties of ovine adipose tissue covering the gluteus muscles as related to pressure ulcer modeling. 114 References [115] Hendriks, F. M., Brokken, D., van Eemeren, J. T. W. M., Oomens, C. W. J., Baaijens, F. P. T., and Horsten, J. B. A. M., (2003). A numerical-experimental method to characterize the non-linear mechanical behaviour of human skin. Skin Research and Technology, 274-283, 9(3), 274-283. [116] Smith, S. R., Lovejoy, J. C., Greenway, F., Ryan, D., deJonge, L., de la Bretonne, J., Volafova, J., and Bray, G. A., (2001). Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism-Clinical and Experimental, 425435, 50(4), 425-435. [117] Bray, G. A., Lovejoy, J. C., Most-Windhauser, M., Smith, S. R., Volaufova, J., Denkins, Y., de Jonge, L., Rood, J., Lefevre, M., Eldridge, A. L., and Peters, J. C., (2002). A 9-mo randomized clinical trial comparing fat-substituted and fat-reduced diets in healthy obese men: the Ole Study. Am.J.Clin.Nutr., 928-934, 76(5), 928934. [118] Lovejoy, J. C., Smith, S. R., and Rood, J. C., (2001). Comparison of regional fat distribution and health risk factors in middle-aged white and African American women: The Healthy Transitions Study. Obes.Res., 10-16, 9(1), 10-16. [119] Krouskop, T. A., Wheeler, T. M., Kallel, F., Garra, B. S., and Hall, T., (1998). Elastic moduli of breast and prostate tissues under compression. Ultrasonic Imaging, 260-274, 20(4), 260-274. [120] sarvazyan, A. P., Skovoroda, A., and Emelianov, S, (1995). Biophysical bases of elasticity imaging. Acoustic Imaging, 223-240, 21223-240. [121] Linder-Ganz, E., Shabshin, Noga, Itzchak, Yacov, and Gefen, Amit, (2007). Assessment of mechanical conditions in sub-dermal tissues during sitting: A combined experimental-MRI and finite element approach. Journal of Biomechanics, 1443-1454, 40(7), 1443-1454. [122] Klein, J., Permana, P. A., Owecki, M., Chaldakov, G. N., Bohm, M., Hausman, G., Lapiere, C. M., Atanassova, P., Sowinski, J., Fasshauer, M., Hausman, D. B., Maquoi, E., Tonchev, A. B., Peneva, V. N., Vlachanov, K. P., Fiore, M., Aloe, L., Slominski, A., Reardon, C. L., Ryan, T. J., and Pond, C. M., (2007). What are subcutaneous adipocytes really good for ...? Experimental Dermatology, 45-70, 16(1), 45-70. [123] Beynen, A. C. and Katan, M. B., (1985). Rapid Sampling and Long-Term Storage of Subcutaneous Adipose-Tissue Biopsies for Determination of Fatty-Acid Composition. American Journal of Clinical Nutrition, 317-322, 42(2), 317-322. References 115 [124] Tirrel, M., (1994). Rheology of Polymeric Liquids. In: Macosko, C. W., Rheology: principles, measurements, and applications. Wiley-VCH, Inc, New york. [125] Hrapko, M., Dommelen, J. A. W. v., Peters, G. W. M., and Wismans, J. S. H. M.,(2005). The mechanical behaviour of brain tissue: large strain response and constitutive modeling. 59-69. 59-69. [126] van Turnhout, M., Peters, G., Stekelenburg, A., and Oomens, C., (2005). Passive transverse mechanical properties as a function of temperature of rat skeletal muscle in vitro. Biorheology, 193-207, 42(3), 193-207. [127] Verbeke, G. and Molenberghs, G. (2000). Linear Mixed Models for Longitudinal data. Springer, New York. [128] Pipkin, A. C. (1986). Lectures on Viscoelasticity. Springer-Verlag, New York. [129] Tanner, R. I. (2000). Engineering Rheology. Oxford University Press, [130] Geerligs, M., Peters, G. W., Ackermans, P. A., Oomens, C. W., and Baaijens, F. P., (2008). Linear viscoelastic behavior of subcutaneous adipose tissue. Biorheology, 677-688, 45(6), 677-688. [131] Darling, E. M., Topel, M., Zauscher, S., Vail, T. P., and Guilak, F., (2008). Viscoelastic properties of human mesenchymally-derived stem cells and primary osteoblasts, chondrocytes, and adipocytes. J.Biomech., 454-464, 41(2), 454-464. [132] Then, C., Menger, J., Benderoth, G., Alizadeh, M., Vogl, T. J., Hubner, F., and Silber, G., (2007). A method for a mechanical characterisation of human gluteal tissue. Technol.Health Care, 385-398, 15(6), 385-398. [133] van Dam, E. A., Dams, S. D., Peters, G. W., Rutten, M. C., Schurink, G. W., Buth, J., and van de Vosse, F. N., (2008). Non-linear viscoelastic behavior of abdominal aortic aneurysm thrombus. Biomech.Model.Mechanobiol., 127-137, 7(2), 127-137. [134] Mewis, J. and Wagner, N. J., (2009). Thixotropy. Advances in Colloid and Interface Science, 214-227, 147-48214-227. [135] Mewis, J. and Wagner, Norman J.Thixotropy. Advances in Colloid and Interface Science, In Press, Corrected Proof [136] O'Neill, P. L. and Stachowiak, G. W., (1996). The inverse thixotropic behaviour of synovial fluid and its relation to the lubrication of synovial joints. Journal of Orthopaedic Rheumatology, 222-228, 9(4), 222-228. 116 References [137] Linder-Ganz, E. and Gefen, A., (2009). Stress analyses coupled with damage laws to determine biomechanical risk factors for deep tissue injury during sitting. J.Biomech.Eng, 011003, 131(1), 011003. [138] Stekelenburg, A., Gawlitta, D., Bader, D. L., and Oomens, C. W., (2008). Deep tissue injury: how deep is our understanding? Arch.Phys.Med.Rehabil., 1410-1413, 89(7), 1410-1413. [139] Avram, M. M., Avram, A. S., and James, W. D., (2005). Subcutaneous fat in normal and diseased states: 1. Introduction. Journal of the American Academy of Dermatology, 663-670, 53(4), 663-670. [140] Palero, J. A., de Bruijn, H. S., van der Ploeg van den Heuvel, Sterenborg, H. J., and Gerritsen, H. C., (1-8-2007). Spectrally resolved multiphoton imaging of in vivo and excised mouse skin tissues. Biophys.J., 992-1007, 93(3), 992-1007. [141] OECD,(13-4-2009). OECD Guideline for the testing of chemicals. Skin absorption: in vitro Method. Test Guideline 428( [142] Garo, A., Hrapko, M., van Dommelen, J. A., and Peters, G. W., (2007). Towards a reliable characterisation of the mechanical behaviour of brain tissue: The effects of post-mortem time and sample preparation. Biorheology, 51-58, 44(1), 51-58. [143] Cox, M. A. J., Driessen, N. J. B., Boerboorn, R. A., Bouten, C. V. C., and Baaijens, F. P. T., (2008). Mechanical characterization of anisotropic planar biological soft tissues using finite indentation: Experimental feasibility. Journal of Biomechanics, 422-429, 41(2), 422-429. [144] Hendriks, F. M., Oomens, C. W. J., Baaijens, F. P. T., and Brokken, D., (2004). Influence of hydration and experimental length scale on the mechanical response of human skin in vivo, using optical coherence tomography. Skin Research and Technology, 231-241, 10(4), 231-241. [145] Bronneberg, D. (2007). Biochemical markers for early detection of superficial pressure ulcers. Ph.D. thesis, Technische Universiteit Eindhoven. [146] cornelissen, l. h. (2008). Modeling the transport of biochemical markers in skin: towards pressure ulcer assessment. Ph.D. thesis, Technische Universiteit Eindhoven. [147] Gawlitta, D. (2006). Compression-induced factors influencing the damage of engineered skeletal muscle. Ph.D. thesis, PrFont34Bin0BinSub0Frac0Def1Margin0Margin0Jc1Indent1440Lim0Lim1Width1 WidthB3WidthA3Width3Width1409tensileWidth1WidthB3WidthA3Width3Width 1409Technische Universiteit Eindhoven. References 117 [148] Bosboom, E. M. H. (2001). Deformation as a trigger for pressure sore related muscle damage. Ph.D. thesis, Technische Unievrsiteit Eindhoven. [149] Stekelenburg, A. (2005). Mechanisms associated with deep tissue injury induced by sustained compressive loading. Ph.D. thesis, Technische Universiteit Eindhoven. [150] Ceelen, K. K. (2008). Modeling the development of in vitro and in vivo pressureinduced muscle damage. Ph.D. thesis, [151] Dullaert, K. and Mewis, J., (2005). A model system for thixotropy studies. Rheologica Acta, 23-32, 45(1), 23-32. [152] Dullaert, K. and Mewis, J., (2005). Thixotropy: Build-up and breakdown curves during flow. Journal of Rheology, 1213-1230, 49(6), 1213-1230.