Chapter 7 Homework
advertisement
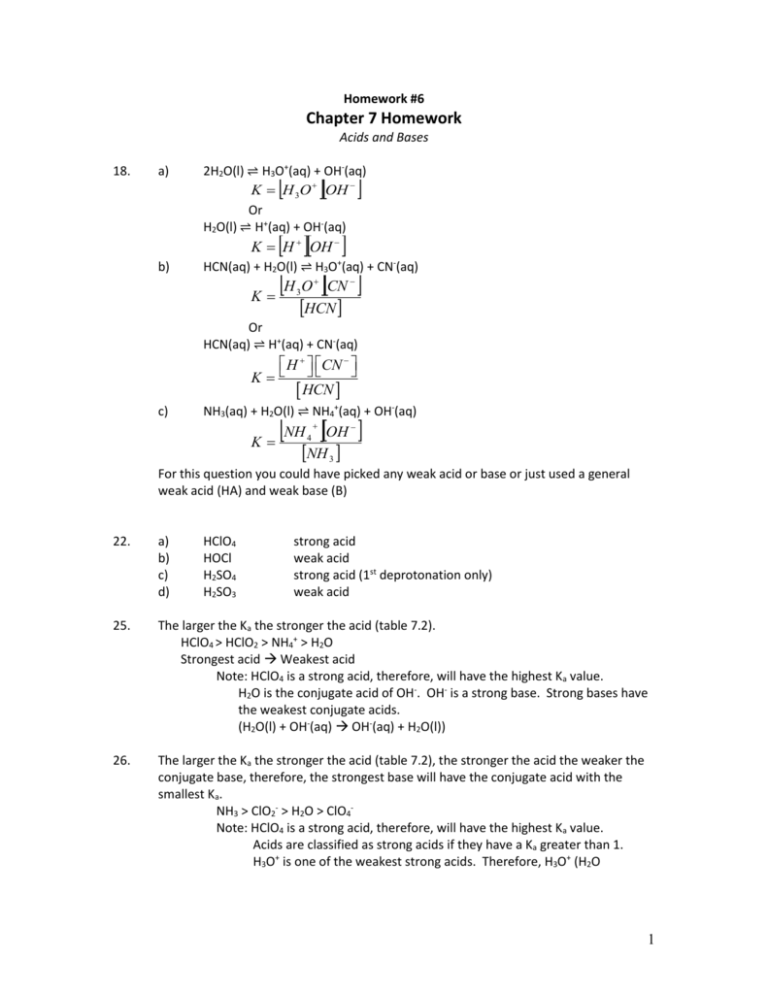
Homework #6 Chapter 7 Homework Acids and Bases 18. a) 2H2O(l) ⇌ H3O+(aq) + OH-(aq) K H 3O OH Or H2O(l) ⇌ H+(aq) + OH-(aq) K H OH b) HCN(aq) + H2O(l) ⇌ H3O (aq) + CN-(aq) + H O CN K 3 HCN Or HCN(aq) ⇌ H+(aq) + CN-(aq) H CN K HCN c) NH3(aq) + H2O(l) ⇌ NH4+(aq) + OH-(aq) NH OH K 4 NH 3 For this question you could have picked any weak acid or base or just used a general weak acid (HA) and weak base (B) 22. a) b) c) d) HClO4 HOCl H2SO4 H2SO3 strong acid weak acid strong acid (1st deprotonation only) weak acid 25. The larger the Ka the stronger the acid (table 7.2). HClO4 > HClO2 > NH4+ > H2O Strongest acid Weakest acid Note: HClO4 is a strong acid, therefore, will have the highest Ka value. H2O is the conjugate acid of OH-. OH- is a strong base. Strong bases have the weakest conjugate acids. (H2O(l) + OH-(aq) OH-(aq) + H2O(l)) 26. The larger the Ka the stronger the acid (table 7.2), the stronger the acid the weaker the conjugate base, therefore, the strongest base will have the conjugate acid with the smallest Ka. NH3 > ClO2- > H2O > ClO4Note: HClO4 is a strong acid, therefore, will have the highest Ka value. Acids are classified as strong acids if they have a Ka greater than 1. H3O+ is one of the weakest strong acids. Therefore, H3O+ (H2O 1 conjugate base) is one of the weakest strong acid and H2O will be slightly more basic than ClO4-. 27. The larger the Ka the stronger the acid. a) HCl strong acid and H2O is a weak acid Kw = Ka (1.0×10-14) b) HNO2 The Ka of HNO2 (4.0×10-4) > Kw = Ka (1.0×10-14) c) HCN The Ka HCN (6.2×10-10) > Ka of HOC6H5 (1.6×10-10) 28. The higher the Ka the weaker the conjugate base a) Clconjugate base of a strong acid, HCl H2O conjugate base of one of the weakest strong acids H3O+ H2O stronger base b) H2O conjugate base of one of the weakest strong acids H3O+ NO2conjugate base of a weak acid NO2- stronger base c) CNconjugate base of the weak acid HCN (Ka = 6.2×10-10) OC6H5conjugate base of the weak acid HOC6H5 (Ka = 1.6×10-10) OC6H5 stronger base 29. a) b) c) CH3CO2H acid H2O base CH3CO2 conjugate base H3O+ conjugate acid Therefore, H2O and CH3CO2- are competing for protons. CH3CO2- is the stronger base (H3O+ is a strong acid, therefore, forms a weaker conjugate acid than CH3CO2H) CH3CO2- is a weak base because it is the conjugate base of a weak acid. Conjugate bases of weak acid are also weak, therefore, the do no fully dissociate in solution. CH3CO2- + H2O(aq) CH3CO2H(aq) + OH-(aq) 31. a) d) g) 36. The solution is acidic if [H+]>[OH-] , the solution is basic if [H+]<[OH-], and the solution is neutral if [H+]=[OH-] . The relationship between [H+] and [OH-] is: K w H OH 1.0 1014 a) weak acid strong base weak acid b) e) h) 1.0 10 OH strong acid weak base strong base c) f) i) weak base weak acid strong acid (1st deprotonation) 1.0 1014 OH 1.0 107 M 7 [H+] = [OH-] neutral solution b) 8.310 OH 16 1.0 1014 OH 12M [H+] < [OH-] basic solution 2 c) 12 OH 1.0 1014 OH 8.3 1016 M [H+] > [OH-] acidic solution 5.4 10 OH 1.0 1014 OH 1.9 1010 M 5 d) [H+] > [OH-] acidic solution 37. If the pH < 7 the solution is acidic, if the pH > 7 the solution is basic, and if the pH = 7 the solution is neutral. If the pOH < 7 the solution is basic, if the pOH > 7 the solution is acidic, and if the pOH = 7 the solution is neutral. The relationship between pH and [H+] is: pH log H The relationship between [H+] and [OH-] is: a) Basic H 10 pH 107.4 4.0 108 M 4.0 10 OH 8 b) 1.0 1014 OH 2.5 107 M Basic H 10 pH 1015.3 5 1016 M 5 10 OH 16 c) K w H OH 1.0 1014 1.0 1014 OH 20M Acidic H 10 pH 101.0 10M 10 OH 1.0 1014 d) OH 11015 M Acidic H 10 pH 103.20 6.3 104 M 2.2 10 OH 4 e) 1.0 1014 OH 1.6 1011 M Basic OH 10 pOH 105.0 1105 M H 1106 1.0 1014 f) H 1109 M Acidic 3 OH 10 pOH 109.60 2.5 1010 M H 1.5 1010 1.0 1014 H 4.0 105 M 43. a) HNO2 is a weak acid The major species in solution are HNO2 and H2O The following equilibrium will occur in solution HNO2(aq) ⇌ NO2-(aq) + H+(aq) Ka = 4.0×10-4 (Table 7.2) + Calculate the concentration of H ions at equilibrium HNO2 NO2H+ 0.250 M 0 0 Initial -x +x +x Change 0.250-x X X Equilibrium NO2 H Ka 4.0 10 HNO2 4 xx 0.250 x 1.0 10 4 4.0 10 4 x x 2 x 2 4.0 10 4 x 1.0 10 4 0 4 b b 2 4ac 4.0 10 x 2a 4.0 10 4 2 4.0 10 4 2 0.0098 and 0.010 x is 0.0098 M because concentration cannot be negative. H x 0.0098M Calculate pH pH log H log0.0098 2.01 b) HC2H3O2 is a weak acid The major species in solution HC2H3O2 and H2O The following equilibrium will occur in solution HC2H3O2(aq) ⇌ C2H3O2-(aq) + H+(aq) Ka = 1.8×10-5 (Table7.2) + Calculate the concentration of H ions at equilibrium HC2H3O2 C2H3O2H+ 0.250 M 0 0 Initial -x +x +x Change 0.250-x X X Equilibrium C2 H 3O2 H Ka 1.8 10 HC2 H3O2 5 xx 0.250 x Since Ka is small assume 0.250-x = 0.250 1.8 10 5 xx 0.250 x 0.0021 Check assumption (5% rule) 4 Amount Gained/Lost 0.0021 100% 100% 0.84% Original Concentration 0.25 Less than 5%, therefore, good assumption H x 0.0021M Calculate pH pH log H log0.0021 2.68 44. a) HOC6H5 is a weak acid therefore The major species in solution are HOC6H5 The following equilibrium will occur in solution HOC6H5(aq) ⇌ OC6H5-(aq) + H+(aq) 𝐾𝑎 = 1.6 × 10−10 + Calculate the concentrations of H at equilirium HOC6H5 OC6H5H+ 0.250 M 0 0 Initial -x +x +x Change 0.250 – x x x Equilibrium OC6 H 5 H Ka 1.6 10 HOC6 H5 10 xx 0.250 x Since Ka is small assume 0.250-x = 0.250 xx 1.6 10 10 0.250 x 6.3 106 Check assumption (5% rule) Amount Gained/Lost 6.3 106 100% 100% 0.0025% Original Concentration 0.250 Less than 5%, therefore, good assumption. H x 6.3 106 M Calculate the concentration of OH- from water equilibrium 1.5 10 OH 1.0 10 OH 6.7 10 M K w H OH 1.0 10 14 5 14 10 Calculate pH pH log H log 6.3 106 5.20 b) HCN is a weak acid The major species in solution are HCN and H2O The following equilibrium will occur in solution HCN(aq) ⇌ CN-(aq) + H+(aq) Ka = 6.2×10-10 (Table 7.2) 5 Calculate the concentration of H+ ion at equilibrium HCN CNH+ 0.250 M 0 0 Initial -x +x +x Change 0.250-x x x Equilibrium CN H Ka 6.2 10 HCN 10 xx 0.25 x Since Ka is small assume 0.250-x = 0.250 6.2 10 10 xx 0.250 x 1.2 10 5 Check assumption (5% rule) Amount Gained/Lost 1.2 105 100% 100% 0.0048% Original Concentration 0.25 Less than 5%, therefore, good assumption. H x 1.2 105 M Calculate pH pH log H log 1.2 10 5 4.92 45. HF is a weak acid therefore, the reactions that are occurring in solution are: HF(aq) ⇌ F-(aq) + H+(aq) Calcualte the concentrations of HF, F-, and H+ at equilibrium (you will also need to calculate the consentration of OH- which will be in the soltion from the following equilibrium H2O(l) ⇌ OH-(aq) + H+(aq)) HF FH+ 0.020 M 0 0 Initial -x +x +x Change 0.020-x x x Equilibrium F H Ka 7.2 10 HF 4 xx 0.020 x 1.44 10 5 7.2 10 4 x x 2 x 2 7.2 10 4 x 1.44 10 5 0 4 b b 2 4ac 7.2 10 x 2a 7.2 10 4 2 5.76 10 5 2 0.0035 and 0.0042 x is 0.030 M because concentration cannot be negative. Find the final concentrations HF 0.020 x 0.020 0.0035 0.017M F H x 0.0035M Calculate the concentration of OH- from water equilibrium 6 0.0035OH 1.0 10 OH 2.9 10 M K w H OH 1.0 10 14 14 12 Calculate pH pH log H log0.0035 2.46 47. a) b) HA is a weak acid. There are more HA molecules (green and white atoms together) than H+ ions (single white atom) and A- ions (single green atoms). This shows that the acid did not completely dissociate making it a weak acid. Calculate the percentage dissociated In the picture there are 9 particles that have note dissociated and 1 particle that has 𝑝𝑎𝑟𝑡𝑖𝑐𝑙𝑒𝑠 𝑑𝑖𝑠𝑠𝑜𝑐𝑖𝑎𝑡𝑒𝑑 1 100% = 100% = 10% 𝑡𝑜𝑡𝑎𝑙 𝑝𝑎𝑟𝑡𝑖𝑐𝑙𝑒𝑠 1+9 Calculate the equilibrium constant The reaction of interest is HA(aq) ⇌ H+(aq) + A-(aq) HA H+ A0.20 M 0 0 Initial -x +x +x Change 1 1 9 Equilibrium 0.20− 𝑥= 𝑥= 𝑥= 𝑁𝑎 𝑉 𝑁𝑎 𝑉 𝑁𝑎 𝑉 9 9 Note from the picture at equilibrium the concentration of HA is 𝑁𝑉𝑎 = 𝑁 𝑎𝑉 Na is Avogadro’s number and is used to convert the 9 particles to number of moles of 1 HA. The concentration of H+ and A- are 𝑁𝑎 𝑉 Solve for V 9 0.20 − 𝑥 = 𝑁𝐴 𝑉 1 9 0.20 − = 𝑁𝑎 𝑉 𝑁𝑎 𝑉 10 50 𝑉= = 𝑁𝑎 0.20 𝑁𝑎 Solve for K 𝐾= 48. [𝐻 + ][𝐴− ] [𝐻𝐴] = 1 1 50 50 𝑁𝑎 (𝑁 )𝑁𝑎 (𝑁 ) 𝑎 𝑎 9 50 𝑁𝑎 ( ) 𝑁𝑎 1 = (50)(9) = 0.0022 HC2H2ClO2 is a weak acid, therefore, the equilibrium established is: HC2H2ClO2(aq) ⇌C2H2ClO2-(aq) + H+(aq) Find the concentration of H+ at equilirbium HC2H2ClO2 C2H2ClO2H+ 0.10 M 0 0 Initial -x x x Change 0.10-x x x Equilibrium 7 C2 H 2 ClO2 H Ka 1.35 10 HC2 H 2ClO2 3 xx 0.10 x 1.4 10 4 1.35 10 3 x x 2 x 2 1.35 10 3 x 1.4 10 4 0 3 3 4 b b 2 4ac 1.35 10 1.35 10 5.6 10 x 0.011 and 0.013 2a 2 2 x is 0.011 M because concentration cannot be negative. H x 0.011M Calculate pH pH log H log0.011 1.96 49. HIO3 is a weak acid, therefore, the equilibrium established is: HIO3(aq) ⇌IO3-(aq) + H+(aq) Find the concentration of H+ at equilibrium HIO3 IO3H+ 0.010 M 0 0 Initial -x X x Change 0.010-x X x Equilibrium IO3 H xx Ka 0.17 0.010 x HIO3 0.0017 0.17 x x 2 x 2 0.17 x 0.0017 0 b b 2 4ac 0.17 x 2a 0.17 2 0.0068 2 0.0094 and 0.18 x is 0.0094 M because concentration cannot be negative. H x 0.0094M Calculate pH pH log H log0.0094 2.03 51. g M C6 H5CO2 H 122.13 mol 0.56 g C6 H 5CO2 H M 1mol C6 H 5CO2 H 122.13 g C6 H 5CO2 H 0.0046mol C H CO H 6 5 2 n 0.0046mol 0.0046M V 1.0 L C6H5CO2H is a weak acid, therefore, the equilibrium established is: H2O(l) ⇌ OH-(aq) + H+(aq) C6H5CO2H(aq) ⇌C6H5CO2-(aq) + H+(aq) 8 Calcualte the equilirium concnetrations C6H5CO2H C6H5CO20.0046 M 0 Initial -x +x Change 0.0046-x X Equilibrium C6 H5CO 2 H Ka 6.4 10 C6 H5CO2 H 5 H+ 0 +x X xx 0.0046 x 2.9 10 7 6.4 10 5 x x 2 x 2 6.4 10 5 x 2.9 10 7 0 5 b b 2 4ac 6.4 10 x 2a 6.4 10 5 2 1.2 10 6 2 5.2 104 and 5.8 104 x is 5.2×10-4 M because concentration cannot be negative. Find the final concentrations C6 H5CO2 H 0.0046 x 0.0046 5.2 104 0.0041M C6 H5CO2 H x 5.2 104 M Calculate the concentration of OH- from water equilibrium 5.2 10 OH 1.0 10 OH 1.9 10 M K w H OH 1.0 10 14 4 14 11 Calculate pH pH log H log 5.2 10 4 3.28 52. C6H5CO2H is a solid before it is put into water. Therefore, two equilibriums are established. C6H5CO2H(s) ⇌ C6H5CO2H(aq) C6H5CO2H(aq) ⇌ C6H5CO2-(aq) + H+(aq) They want to know how much C6H5CO2H will dissolve. This will be the amount of C6H5CO2H that starts in the second equilibrium which we will denote as s. C6H5CO2H C6H5CO2H+ S 0 0 Initial -x +x +x Change s-x x x Equilibrium The pH of the solution will give us the equilibrium concentration of the H+ ions pH log H H 10 pH Initial Change Equilibrium 10 2.80 0.0016M C6H5CO2H s -0.0016 s-0.0016 C6H5CO20 +0.0016 0.0016 H+ 0 +0.0016 0.0016 9 C6 H 5CO2 H 0.0016 0.0016 Ka 6.4 105 S 0.0016 C6 H 5CO2 H S 0.042M Convert to grams per 100 ml 0.042𝑚𝑜𝑙 1𝐿 122.13𝑔 𝑔 𝑆 = ( 1𝐿 ) (1000𝑚𝐿) ( 1𝑚𝑜𝑙 ) = 0.0051 𝑚𝐿 Therefore the water solubility per 100 ml is 0.51 g per 100 ml 55. a) The first solution contains 0.10 M HCl (strong acid) and 0.10 M HOCl (weak acid). Since there is both a strong and weak acid with equal concentrations of each the number of H+ ions from the weak acid will be minimal therefore the pH will be completely from the strong acid (𝑝𝐻 = − log[𝐻 + ] = − log 0.10 = 1.00 This can be proven using an ICE table HOCl(aq) ⇌ H+(aq) + OCl-(aq) 𝐾𝑎 = 3.5 × 20−8 HOCl OClH+ 0.10 M 0 0.10 Initial -x +x +x Change 0.10-x x 0.10+x Equilibrium OCl H x 0.10 x Ka 3.5 10 8 0.10 x HOCl Since Ka is small assume 0.250-x = 0.250 x 0.10 3.5 10 8 0.10 x 3.5 108 Check assumption (5% rule) Amount Gained/Lost 3.5 108 100% 100% 3.5 105% Original Concentration 0.10 Less than 5%, therefore, good assumption. H 0.10 x 0.10 3.5 108 0.10M Calculate pH pH log H log 0.10 1.00 b) The second solution contains 0.050 M HNO3 (strong acid) and 0.50 M HC2H3O2 (weak acid). Since there is both a strong and weak acid and even though the concentration of the weak acid is 10x less than the concentration of the strong acid the number of H+ ions from the weak acid will be minimal therefore the pH will be completely from the strong acid (𝑝𝐻 = − log[𝐻 + ] = − log 0.050 = 1.30 This can be proven using an ICE table HC2H3O2(aq) ⇌ H+(aq) + C2H3O2-(aq) 𝐾𝑎 = 1.8 × 20−5 HC2H3O2 C2H3O2H+ 0.50 M 0 0.050 Initial -x +x +x Change 0.50-x X 0.050+x Equilibrium 10 C2 H 3O2 H x 0.050 x Ka 1.8 10 5 0.50 x HC2 H3O2 Since Ka is small assume 0.50-x = 0.50 and 0.05+x=0.05 x 0.05 1.8 10 5 0.50 x 1.8 104 Check assumption (5% rule) Note:0.05<0.50 therefore is the assumption works for 0.05-x then it will work form 0.50-x Amount Gained/Lost 1.8 104 100% 100% 0.36% Original Concentration 0.05 Less than 5%, therefore, good assumption. H 0.050 x 0.050 1.8 104 0.050 Calculate pH pH log H log 0.50 1.30 59. HOCN is a weak acid, therefore, the following equilibrium is established: HOCN(aq) ⇌ OCN-(aq) + H+(aq) HOCN OCNH+ 1.00×10-2 M 0 0 Initial -x +x +x Change -2 1.00×10 -x x x Equilibrium The pH of the solution at equilibrium will allow us to calculate the concentration of H+ ions at equilibrium =x pH log H H 10 pH 10 2.77 0.0017 Updating the ICE table gives HOCN 1.00×10-2 M Initial -0.0017 Change 0.0083 Equilibrium Ka 63. OCN0 +0.0017 0.0017 H+ 0 +0.0017 0.0017 OCN H 0.00170.0017 3.5 10 HOCN 0.0083 4 The larger the Kb the stronger the base. Therefore the strongest base will have the largest Kb. NH3 > C5H5N > H2O > NO3Strongest base Weakest base Note: HNO3 is a strong acid. The stronger the acid the weaker the conjugate base. Therefore, NO3- is a very weak base. Note: H2O is the conjugate base of (H3O+). H3O+ is one of the weakest strong acids. Therefore, H2O is only slightly more basic than NO3- 11 64. The smaller the Kb the stronger the conjugate acid. HNO3 > C5H5NH+ > NH4+ > H2O Strongest acid Weakest acid Note: HNO3 is a strong acid. Note: C5H5N and NH3 are both weak bases, therefore, will have weak conjugate acids. The Kb of NH3 > kb of C5H5N resulting in C5H5NH+ being a stronger acid than NH4+. Note: H2O is the conjugate acid of OH-. OH- is a strong base. Strong bases have the weakest conjugate acids. (H2O(l) + OH-(aq) OH-(aq) + H2O(l)) 68. a) Ca(OH)2 is a strong base (fully dissociates) OH 0.00040M Ca(OH ) 2 2 mol Ca ( OH )2 1mol Ca ( OH )2 0.00080M OH pOH log OH log 0.00080 3.10 pH pOH 14 pH 14 pOH 14 3.10 10.90 b) KOH is a strong base (fully dissociates) g M KOH 56.11 mol 25 g KOH 1mol KOH 56.11 g KOH 1mol OH 1mol KOH 0.45mol OH n 0.45mol OH 0.45M V 1L pOH log OH log 0.45 0.35 pH pOH 14 pH 14 pOH 14 0.35 13.65 c) NaOH is a strong base (fully dissociates) g M Na 5OH 40.00 mol 150.0 g NaOH 1mol NaOH 40.00 g NaOH 1mol OH 1mol NaOH 3.750mol OH n 3.750mol OH 3.750M V 1L pOH log OH log 3.750 0.5740 pH pOH 14 pH 14 pOH 14 0.5740 14.5740 12 69. Ba(OH)2 is a strong base (fully dissociates) Calculate the concentration of OH- 14 pH pOH pOH 14 pH 14 10.50 3.50 pOH log OH OH 10 pOH 10 3.50 3.2 10 4 Calculate the concentration of Ba(OH)2 3.2 104 M OH 1mol Ba OH 2 2 mol OH 1.6 10 4 M Ba OH 2 73. The presence of nitrogen most often results in a basic molecule. Nitrogen has an unpaired electron, therefore, bonds easily to H+ ions. 93. The larger the pH the more basic a solution is If a salt is made from Acid Base the solution will be Strong Acid Strong Base Neutral Strong Acid Weak Base Acidic Weak Acid Strong Base Basic Ka > Kb Acidic Weak Acid Weak Base Ka < Kb Basic Ka = Kb Neutral a) HI strong acid HF weak acid NaF weak base (a salt formed from strong base and weak acid) NaI neutral (a salt formed from a strong base and a strong acid) HI < HF < NaI < NaF Increasing pH b) NH4Br weak acid (a salt formed from a weak base and strong acid) HBr strong acid KBr neutral (a salt formed from a strong base and a strong acid) NH3 weak base HBr < NH4Br < KBr < NH3 Increasing pH c) C6N5NH3NO3 weak acid (a salt formed from a weak base and a strong acid) NaNO3 neutral (a salt formed from a strong base and a strong acid) NaOH strong base HOC6H5 weak acid (Ka = 1.6×10-10) KOC6H5 weak base (a salt formed from a strong base and a weak acid) C6H5NH2 weak base HNO3 Strong acid There are 2 weak acid C6N5NH3NO3 (C6H5NH3+) and HOC6H5 Compare the Ka values to determine the weaker acid HOC6H5 Ka = 1.6×10-10 13 The Ka value of C6H5NH3+ is not given therefore, it must be calculated C6H5NH2(aq) Kb = 3.8×10-10 K w 1.0 1014 Ka 2.6 105 10 Kb 3.8 10 Therefore, C6N5NH2NO3 is a stronger acid than HOC6H5 There are 2 weak bases C6N5NH2 and KOC6H5 (OC6H5-) Compare the Kb values to determine the weaker base C6N5NH2 Kb = 3.8×10-10 The Kb value of OC6H5- is not given therefore, it must be calculated HOC6H5(aq) Ka = 1.6×10-10 Kb K w 1.0 1014 6.2 105 Kb 1.6 1010 Therefore, KOC6H5 is a stronger base than C6N5NH2 HNO3< C6N5NH3NO3 < HOC6H5 < NaNO3< C6H5NH2 < KOC6H5 < NaOH 95. See chart for problem 85. a) Sr(NO3)2 (salt formed form a strong acid and strong base) b) C2H5NH3CN (a salt formed from a weak base and a weak acid need to compare Ka and Kb) We are not given the Ka of C2H5NH3+, therefore, we need to calculate it C2H5NH2(aq) Kb = 5.6×10-4 Ka K w 1.0 1014 1.8 1011 4 Kb 5.6 10 We are not given the Kb of CN-, therefore, we need to calculate it HCN(aq) Ka = 6.2×10-10 Kb c) K w 1.0 1014 1.6 105 K a 6.2 1010 Since Ka < Kb the solution will be basic C5H5NHF (a salt formed from a weak base and a weak acid need to compare Ka and Kb) We are not given the Ka of C5H5NH+, therefore, we need to calculate it C2H5N(aq) Kb = 1.7×10-9 K w 1.0 1014 Ka 5.9 106 9 Kb 1.7 10 We are not given the Kb of F-, therefore, we need to calculate it HF(aq) Ka = 7.2×10-4 Kb d) K w 1.0 1014 1.4 1011 4 K a 7.2 10 Since Ka > Kb the solution will be acidic NH4C2H3O2 (a salt formed from a weak base and a weak acid need to compare Ka and Kb) NH4+ Ka = 5.6×10-10 14 We are not given the Kb of C2H3O2- therefore we need to calculate it HC2H3O2(aq) Ka = 1.8×10-5 K w 1.0 1014 Kb 5.6 1010 5 K a 1.8 10 e) Ka = Kb neutral solution NaHCO3 (a salt formed from a strong base and a weak acid) suggest basic but this is a polyprotic acid there must look at Na+ being a spectator ion and HCO3- being a weak acid. Therefore need to compare Ka and Kb values HCO3Ka = 4.8×10-11 We are not given the Kb of HCO3 therefore we need to calculate it H2CO3(aq) Ka = 4.3×10-7 Kb K w 1.0 1014 2.3 108 7 K a 4.3 10 Since Ka < Kb basic solution 97. a) b) KCl (a salt formed from a strong base and a weak acid) Therefore, the solution will be neutral pH=7.00. For neutral solutions the concentration of H+ and OH- equals 1.0×10-7 M. KF (a salt formed form a strong base and a weak acid) Therefore, expect the solution to e basic Interested in the following reaction F-(aq) + H2O(l) ⇌ HF(aq) + OH-(aq) [𝐻𝐹][𝑂𝐻 − ] 𝐾𝑏 = [𝐹 − ] Problem we do not know Kb HF(aq) Ka=7.2×10-4 𝐾𝑏 = 𝐾𝑤 𝐾𝑎 = 1.0×10−14 7.2×10−4 = 1.4 × 10−11 Calculate the concentration of OH- ions FHF 1.0 M 0 Initial -x +x Change 1.0-x x Equilibrium OH HF xx Kb 1.4 1011 1.0 x F OH0 +x x Since Kb is small assume that 1.0-x = 1.0 xx 1.0 6 x 3.7 10 M 1.4 1011 Check assumption (5% rule) Amount Gained/Lost 3.7 106 100% 100% 3.7 104% Original Concentration 1.0 Less than 5%, therefore, good assumption. 15 Calculate OH- concentration OH x 3.7 106 M Calculate H+ concentration [𝐻 + ][𝑂𝐻− ] = 1.0 × 10−14 1.0 × 10−14 [𝐻 + ] = = 2.7 × 10−9 3.7 × 10−6 Calculate pH pH log H log 2.7 109 M 8.57 101. a) KNO2 (a salt formed from a strong base and a weak acid) Therefore, expect the solution to be basic Interested in the following reaction NO2-(aq) + H2O(l) ⇌ HNO2(aq) + OH-(aq) Kb HNO2 OH NO 2 Problem we don’t know Kb Know HNO2(aq) Kb Ka = 4.0×10-4 K w 1.0 1014 2.5 1011 4 K a 4.0 10 Calculate the concentration of OH- ions NO2HNO2 0.12 M 0 Initial -x +x Change 0.12-x X Equilibrium Kb HNO2 OH NO 2 OH0 +x x xx 2.5 1011 0.12 x Since Kb is small assume that 0.12-x = 0.12 xx 0.12 6 x 1.7 10 M 2.5 1011 Check assumption (5% rule) Amount Gained/Lost 1.7 106 100% 100% 0.0014% Original Concentration 0.12 Less than 5%, therefore, good assumption. Calculate OH+ concentration OH x 1.7 106 M Calculate pH pOH log OH log 1.7 106 5.77 pH pOH 14 pH 14 pOH 14 5.77 8.23 16 b) NaOCl (a salt formed from a strong base and a weak acid) Expect the solution to be basic Interested in the following reaction OCl-(aq) + H2O(l) ⇌ HOCl(aq) + OH-(aq) Kb HOCl OH OCl Problem we don’t know Kb HOCl(aq) Ka = 3.5×10-8 1.0 1014 Kb 2.9 107 8 3.5 10 Calculate the concentration of OH- ions OClHOCl 0.45 M 0 Initial -x +x Change 0.45-x X Equilibrium Kb HOCl OH OCl OH0 +x x xx 2.9 107 0.45 x Since Kb is small assume that 0.45-x = 0.45 xx 0.45 4 x 3.6 10 M 2.9 107 Check assumption (5% rule) Amount Gained/Lost 3.6 104 100% 100% 0.08% Original Concentration 0.45 Less than 5%, therefore, good assumption. Calculate OH+ concentration OH x 3.6 104 M Calculate pH pOH log OH log 3.6 104 3.44 pH pOH 14 pH 14 pOH 14 3.44 10.56 c) NH4OCl4 (a salt from a weak base and a strong acid) Therefore, expect the solution to be acidic Interested in the following reaction NH4+(aq) ⇌ NH3(aq) + H+(aq) K w NH 3 H Ka 5.6 1010 Kb NH 4 17 Calculate the concentration of H+ ions NH4+ 0.40 M Initial -x Change 0.40-x Equilibrium Ka NH3 H NH 4 NH3 0 +x x H+ 0 +x X xx 5.6 1010 0.40 x Since Kb is small assume that 0.40-x = 0.40 xx 0.40 5 x 1.5 10 M 5.6 1010 Check assumption (5% rule) Amount Gained/Lost 1.5 105 100% 100% 0.0038% Original Concentration 0.40 Less than 5%, therefore, good assumption. Calculate H+ concentration H x 1.4 105 M Calculate pH pH log H log 1.5 105 4.82 121. Because the solution has a pH of 4.6 we know that the solution is acidic, therefore, NaOH, NH3, and NaCN cannot be the solute. Because the bulb is bright it means that there are a lot of ions in solution. Therefore, a strong electrolyte is need. Only HCl, NaOH, and NH4Cl are strong electrolytes. NaOH was already eliminated because it was basic. HCl could light the bulb bright because it is a strong acid but the pH of a 1.0 M solution of HCl is pH log H log1.0 0 Confirm the solution is made from NH4Cl by compare actual to calculated Ka values Determine the Ka of the weak acid. HA(aq) ⇌ A-(aq) + H+(aq) Ka = unknown A H Ka HA Initial Change Equilibrium HA 1.0 M -x 1.0-x A0 +x x H+ 0 +x x 18 Determine x 𝑥 = [𝐻 + ] 𝑝𝐻 = − log[𝐻 + ] 4.6 = − log[𝐻 + ] [𝐻 + ] = 2.5 × 10−10 A H 2.5 105 2.5 105 Ka 6.3 1010 5 HA 1.0 2.5 10 This Ka of NH4+ is 5.6×10-10 which is close to the calculated value. Therefore the solution must contain NH4Cl 19








