Molecular Modeling of Covalent Compounds
advertisement

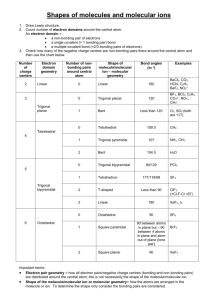
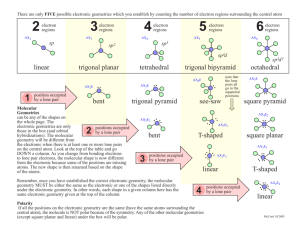
CHEM 121L General Chemistry Laboratory Revision 1.5 Molecular Modeling of Covalent Compounds To learn about the geometry of covalently bound molecules. To learn about VSEPR theory. To learn about Isomerism. To learn about Molecular Polarity. In this laboratory exercise we will build models of some simple molecules that are in accordance with the geometries suggested by the Valence Shell Electron Pair Repulsion (VSEPR) Theory. We will note the influence Lone Pairs of electrons and Multiple Bonds have upon the geometry of these molecules. We will also note the overall polarity of the molecules modeled. VSEPR theory provides a simple extension of Lewis bonding theory; predicting molecular geometries for covalently bound molecules, polyatomic ions and networks. This theory assumes the shape of a molecule is influenced by the number of electron pairs about each central atom in the molecule. Although largely accurate in predicting molecular geometries, it is a bit superficial and must be supplemented with Valence Bond Theory in order to provide a more accurate picture of the orbital structure of said electron pairs. In any case, both VSEPR and Valence Bond Theory are being supplanted by the more robust, although more computationally demanding, Molecular Orbital Theory to describe both the electronic structure and geometry of simple molecules. The basic idea underlying VESPR is that each valence shell electron pair around an atom will mutually repel all the other valence shell electron pairs about that atom. Therefore, the electron pairs and covalent bonds will find a geometric arrangement which minimizes these repulsions. These arrangements, for the common cases, are: Num. Electron Domains 2 3 4 5 6 Geometry Angle Between Domains Linear 180o Trigonal Planar 120o Tetrahedral 109.5o Trigonal Bipyramidal 120o (equatorial) & 90o (axial) Octahedral 90o This Electronic Geometry defines the type of Molecular Geometry possible. For each type of Electronic Geometry, there is a subset of Molecular Geometries which depend on the number of atoms covalently bound to the central atom, as well as the number of Lone Pairs about that atom. As an example, each of the following molecules, CH4, NH3, H2O and HF, is tetrahedral in its Electronic Geometry; each has four electron domains about the central atom. However, they each exhibit different Molecular Geometries. CH4 is tetrahedral but NH3 is trigonal pyramidal; Page |2 CH4 has 4 bonding atoms about the C atom, whereas NH3 has only 3, with the fourth electron pair being a Lone Pair. When determining Molecular Geometries, Lone Pairs of electrons are not considered. The Lone Pairs influence the Molecular Geometry, but do not participate in it. A list of possible Molecular Geometries for 2-6 electron pairs about a central atom E is provided below: Page |3 Several points need to be considered when identifying Molecular Geometries: i) Double and triple bonds count as a single VSEPR domain. This is because a second covalent bond between two atoms shortens and strengthens the overall bonding, but it does not change the geometry of the bonding. For the series CH3CH3, CH2CH2, CHCH, the Carbon-Carbon bond length gets progressively shorter, simply bringing the Carbon atoms closer together. In the example below, the central Carbon atom has three VSEPR domains and so is trigonal planar in its geometry. Page |4 ii) Lone Pair – Lone Pair interactions are more unfavorable than Lone Pair – Bonding Pair interactions. Likewise, these last are more unfavorable than Bonding Pair Bonding Pair interactions. So, Xenon Tetrafluoride (XeF4), which has 4 Bonding Pairs and 2 Lone Pairs about the central Xenon atom, adopts a Square Planar molecular geometry. iii) Molecules with multiple central atoms will have multiple geometries. So, CH3SH has two central atoms; the carbon and sulfur. Thus, it has two specified geometries. It is tetrahedral about the carbon (4 Bonding Pairs) and bent about the sulfur (2 Bonding Pairs and 2 Lone Pairs). iv) The bond angles in a molecule are determined, primarily, by the Electronic Geometry. So, both CH4 and NH3 have approximate bond angles of 109.5o. It is true, the Lone Pair in NH3 requires more space than the Bonding Pairs; the measured bond angle in NH3 is 107o. However, as a first approximation, we can take the bond angles to be those specified by the electron domains listed above. Page |5 Thus, the process for determining the Molecular Geometry of a covalently bound compound is: • • • Write the Lewis Structure for the molecule. Determine the Electronic Geometry of the molecule. Determine the Molecular Geometry. An example is in order. Consider the compound Nitrogen Trichloride, NCl3. A valid Lewis Structure for this compound is: The central Nitrogen atom has four electron domains; three Bonding Pairs and one Lone Pair. This gives rise to a Tetrahedral Electronic Geometry. Four electron domains with one Lone Pair, as can be seen in the Table above, gives rise to a Trigonal Pyramidal Molecular Geometry. Finally, the molecular geometry plays an important role in determining if the molecule is Polar. For instance, both BF3 and GeF2 have very polar bonds. (This is because Fluorine is very Electronegative and the metalloids B and Ge are somewhat Electropositive.) However, BF3, because of its symmetric Trigonal Planar structure, is non-polar, whereas GeF2, adopting an asymmetric Bent structure, is polar. Page |6 In this laboratory exercise, we will build models of molecules for several compounds. This will help us visualize the three dimensional structure of these molecules. It should be emphasized, chemical reactions occur in three dimensions and so inherently depend on the three dimensional structure of the reactants. This is especially important in biochemistry, where enzymes are specifically designed for chemical substrates with a particular three dimensional structure. Page |7 Pre-Lab Questions 1. Draw Lewis Structures for each of the molecules and ions in the Procedure section of the lab. You may skip the Lewis Structure for Morphine. (If you do this now, your time spent in the lab will be much shorter.) Page |8 Procedure We will use two different model kits for the following exercises. The Prentice-Hall Molecular Model Set for General and Organic Chemistry will be used for simpler cases involving atoms that adopt Tetrahedral, Trigonal Planar and Linear Electronic Geometries. It will also be used for molecules that contain multiple geometric centers. For molecules containing Trigonal Bipyramidal and Octahedral centers, the Indigo Instruments Model Kit will be used. For the following cases, check-out and use the Prentice-Hall Molecular Model Set for General and Organic Chemistry. Simple Molecules and Ions For each case below: i) Draw the Lewis Structure of the molecule/ion. ii) Use the molecular modeling kits provided to build a model of the molecule/ion. Use the generally accepted color codes for given elements, as listed on the model kit. Each model must be checked by the instructor. iii) Sketch the Electronic Geometry about the central atom. iv) Sketch the Molecular Geometry about the central atom. v) Label the geometry and bond angles. vi) Determine if the molecule is Polar. (You do not need to do this for the Polyatomic Ions.) Note: Your sketches must use the wedge and dash notation used in the sketches above. SiH4, PH3, H2S, HCl COCl2 (Phosgene), HCN (Hydrogen Cyanide) CO2, CO, NO2, NO NH4+, ClO4Molecules with Multiple Centers For each case below, where the molecule contains multiple centers: i) Draw the Lewis Structure of the molecule. ii) Use the molecular modeling kits provided to build a model of the molecule. Use the Page |9 generally accepted color codes for given elements, as listed on the model kit. Each model must be checked by the instructor. iii) Sketch the Molecular Geometry about each central atom. iv) Label the geometry and bond angles. Note: Your sketches must use the wedge and dash notation used in the sketches above. CH3OH, CH3NH2 CH3CH3, CH2CH2, CHCH Penicillin (R-C9H11N2O4S) i) This structure is as pictured below. Each “point” in the picture is a Carbon atom. Bonds to Carbon not explicitly shown are to Hydrogen. ii) In this structure, "R" represents a variable group which is different from one type of Penicillin to another. For our purposes, we can take R = Hydrogen. P a g e | 10 Isomers Isomers are substances that have the same chemical formula, but are different compounds; i.e., each has its own set of physical and chemical properties. An example is the isomerism exhibited by compounds with the chemical formula C4H8. Two isomers with this formula are: 1-Butene H3C-CH2-CH=CH2 2-Butene H3C-CH=CH-CH3 In the case of 1-Butene, the Carbon-Carbon double bond is between Carbons 1 and 2. For 2-Butene it is between Carbons 2 and 3. Each of these compounds has distinctly different Boiling Points, Melting Points, Densities, etc. This type of Isomerism is a Structural Isomerism; the bonding between the atoms is different from one isomer to another. Other types of isomerism will be covered in your Organic and Inorganic chemistry courses. Build models of the following two structural isomers. Sketch each structure. (Use the standard wedge and dash notation in each sketch.) CH3CH2OH (Grain Alcohol) CH3OCH3 (Dimethyl Ether) For the following cases, check-out and use the Indigo Instruments Model Kit. (Parts are contained in a Red Cup.) Expanded Octets For each case below: i) Draw the Lewis Structure of the molecule/ion. ii) Use the molecular modeling kits provided to build a model of the molecule/ion. Use the generally accepted color codes for given elements, as listed on the model kit. Each model must be checked by the instructor. iv) Sketch the Molecular Geometry about the central atom. Note: Your sketches must use the wedge and dash notation used in the sketches above. PCl5 SF4 Build both possibilities. Explicitly include the Lone Pairs. Which Geometry is better? P a g e | 11 XeF4 Build both possibilities. Explicitly include the Lone Pairs. Which Geometry is better? Resonance Structures In many cases, Lewis Structures do not adequately describe the bonding between atoms in a molecule. This is frequently due to delocalization of the bonding. And, in many cases, this delocalized bonding is due to overlap of unhybridized atomic p-orbitals. The bonding in these cases is usually represented by the use of Resonance Structures. The Indigo Instruments Model Kit contains “lobes” that can be used to represent porbitals. So, for a molecule like Ethene (CH2=CH2), where each Carbon is sp2 hybridized, the unhybridized p-orbitals overlap and form a -bond between the Carbon atoms. CH2=CH2 Build a model of this molecule that explicitly shows the p-orbitals used to construct the -bond. O3 First draw the two resonance structures for this molecule. Then build a model of this molecule that explicitly shows the p-orbitals involved in the delocalized bonding. P a g e | 12 Post Lab Questions 1. Part of the reasoning that led van't Hoff and Le Bel to propose a tetrahedral shape for Methane, CH4, was based on the number of compounds that are theoretically possible for substituted Methanes; that is, compounds in which one or more hydrogens have been replaced by some other group. For example, only one compound of the type CH2X2 has ever been found. Is this consistent with a tetrahedral shape? With a square planar shape? Provide an explanation for each answer using appropriate diagrams. 2. For H2O, the H-O-H bond angle is 104.5o and the measure dipole moment is 1.85 Debye. What is the magnitude of the O-H bond dipole moment for H2O? Estimate the bond angle in H2S given that the H-S bond dipole moment is 0.67 Debye and the resultant molecular dipole moment is 0.95 Debye. 3. There has been some back-and-forth concerning the correct Lewis Structure for the Sulfate Ion; SO42-. At one extreme, the Lewis Structure of SO42- can be represented using a strict adherence to the octet rule. At the other extreme, Sulfur’s valence shell is expanded to allow for a minimization of atomic Formal Charges. Draw the Lewis Structures for Sulfate using both of these extremes. Include Formal Charges for each atom. What geometry is predicted for each of these structures? Some interesting computational results are presented in “Common Textbook and Teaching Misrepresentations of Lewis Structures” by Lalia Suidan, Jay K. Badenhoop, Eric. D. Glendening, and Frank Weinhold Journal of Chemical Education 72 (1995) 583. P a g e | 13 Addendum The Polarity of Water Molecules The shape of the molecules which comprise a substance have a profound effect on the chemical and physical properties of that substance. Certain chemical reactions can depend as heavily on the three dimensional structure of the molecules involved as on the nature of the chemical bonds which must break and reform during the reaction. Additionally, the boiling point, melting point, density, and other physical properties of the substance will depend on the three dimensional structure of the substance's molecules. As a case in point, consider the structure of Water molecules. As we have seen, the molecules which comprise a sample of water have a Bent geometry. This bent structure imparts a strong polarity on the molecule because the polar O-H bonds: contribute to an overall dipole moment for the molecule. These molecular dipole moments, representing a separation of positive and negative electric charge, can be oriented along the field lines emanating from a charged object, such as a charged rod. The resulting Polarization will cause the water sample will be attracted to the charged object. P a g e | 14 There are a few subtleties here,however. First, the orientation of the water molecules due to the presence of the charged object is not complete. Overall, only a few percent of the total number of molecules in the sample will be oriented along the electric field. The polarization is more a skewing of the molecule's orientation along the electric field lines. Second, the polarization of a sample is not limited to those substances with polar molecules. The electron clouds of molecules can polarize when in the presence of an external electric field. This electron cloud polarization generates an induced dipole moment within the molecule. This induced dipole can give rise to Polarization of the sample. However, this polarization is typically much weaker than the polarization that occurs in a sample comprised of polar molecules. In this exercise, we will observe the polarization of Water by bringing a charged object near a narrow stream of water. This will be compared with the rather weak polarization of the nonpolar compound Cyclhexane, C6H12. Procedure 1. A pair of burets, one filled with Water and the other filled with Cyclohexane (C6H12), are available. Water is composed of polar molecules, while Cyclohexane molecules are nonpolar Arrange these so they drain into a large beaker. 2. Allow a stream of Water to flow from the buret into the beaker. 3. Charge a glass rod by briskly rubbing it in your hair or on a woolen garment. This will Charge the rod by Friction; electric charge is transferred from your hair/garment to the rod. (Does it matter what charge is imparted to the rod?) 4. Bring the rod near the stream of Water. Observe the results. 5. Do the same with a buret filled with the non-polar liquid Cyclohexane. 6. Refill the burets with the liquid from the beakers when you are done.