Exam 2 - Chemistry
Exam 2
Name __________________________
CHEM 210
1. (10 pts) Draw both the cis and trans isomer of 1-chloro-3-ethylcyclohexane (pictured), each as a pair of chair conformers. Which isomer is most stable and why?
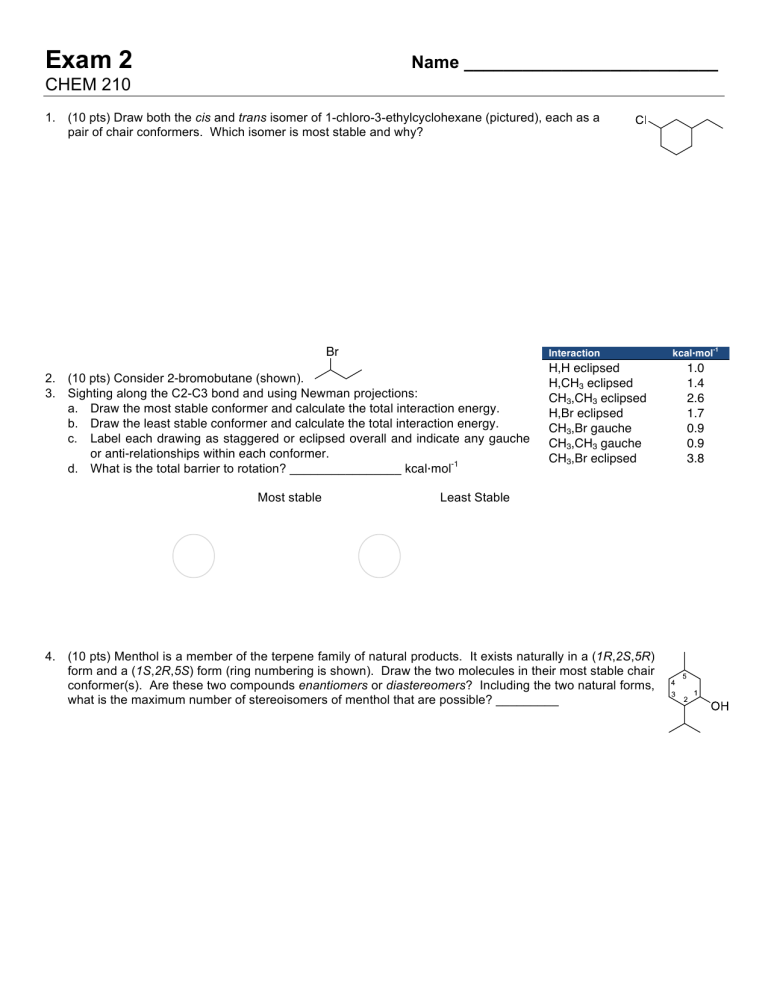
Br Interaction kcal · mol
-1
2. (10 pts) Consider 2-bromobutane (shown).
3. Sighting along the C2-C3 bond and using Newman projections: a. Draw the most stable conformer and calculate the total interaction energy.
b. Draw the least stable conformer and calculate the total interaction energy.
c. Label each drawing as staggered or eclipsed overall and indicate any gauche or anti-relationships within each conformer.
d. What is the total barrier to rotation? ________________ kcal · mol
-1
H,H eclipsed
H,CH
3
eclipsed
CH
3
,CH
3
eclipsed
H,Br eclipsed
CH
3
,Br gauche
CH
3
,CH
3
gauche
CH
3
,Br eclipsed
1.0
1.4
2.6
1.7
0.9
0.9
3.8
Most stable Least Stable
4. (10 pts) Menthol is a member of the terpene family of natural products. It exists naturally in a ( 1R , 2S , 5R ) form and a ( 1S , 2R , 5S ) form (ring numbering is shown). Draw the two molecules in their most stable chair conformer(s). Are these two compounds enantiomers or diastereomers ? Including the two natural forms, what is the maximum number of stereoisomers of menthol that are possible? _________
5. (9 pts) Name the following compounds using IPUAC nomenclature: a.) b.) c.) d.) e.) f.)
Br g.) h.)
OH
OH
6. (9 pts) Draw the following compounds: b.) 3-methyl-2-octanol a.) isobutylcycloheptane c.) 4,4-dimethyl-1-pentyne d.) bicyclo[2.2.2]octane e.) cis -1-iodo-2-butene
7. (10 pts) Assign R or S configurations to all of the chirality centers in the following pharmaceuticals/biomolecules:
Ephedrine – bronchodiolator and decongestant isolated from the Chinese plant ephedra sinica
Biotin – Vitamin B
7
Other copies of the molecules for scratch work (final answers must be on structures above!).
8. (10 pts) The Pasteur separation of the mirror-image crystals of tartaric acid was one of the developments that led to the understanding of chirality in molecules. Draw all stereoisomers for tartaric acid and fully indicate the interrelationships of all (enantiomers, diastereomers or meso).
Be sure to assign R , S configurations to each chiral center.
HO
HO
O O
OH
OH
9. (12 pts) Indicate whether the following pairs of compounds are identical, enantiomers, diastereomers or constitutional isomers.
a.
b.
c.
d.
e.
f.
10. (5 pts) Compare the three staggered conformers of ethylene glycol. The anti conformation is not the lowest energy conformation. The other two staggered conformations are actually lower in energy than the anti conformation. Suggest an explanation.
11.
(5 pts) It is possible for a compound to be chiral even though it lacks a carbon atom with four different groups. For
example, consider the structure of the following allene. Draw its enantiomer and explain why it is chiral.
Exam 2 - KEY
Name __________________________
CHEM 210
1. (10 pts) Draw both the cis and trans isomer of 1-chloro-3-ethylcyclohexane (pictured), each as a pair of chair conformers. Which isomer is most stable and why?
Cl Cl
Cl
H
H cistwo large groups axial - high PE
H H both large groups equitorial - low PE
Cl
H
Cl H
Cl
H transH
One group axial, other equitorial one group equitorial, other axial
2. (10 pts) Consider 2-bromobutane (shown). Sighting along the C2-C3 bond and using Newman projections: e. Draw the most stable conformer and calculate the total interaction energy.
f. Draw the least stable conformer and calculate the total interaction energy.
g. Label each drawing as staggered or eclipsed overall and indicate any gauche or anti-relationships within each conformer.
h. What is the total barrier to rotation? _____6.2 ___________ kcal
· mol
-1 these two conformers are higher in PE than the right hand side of the eqm for cis-. as one large group is always axial. The left side of this is higher in PE as the ethyl group is larger than -Cl cis -1,3 is more stable than trans -1,3
Interaction
H,H eclipsed
H,CH
3
eclipsed
CH
3
,CH
3
eclipsed
H,Br eclipsed
CH
3
,Br gauche
CH
3
,CH
3
gauche
CH
3
,Br eclipsed kcal
· mol
-1
1.0
1.4
2.6
1.7
0.9
0.9
3.8
Staggered
Eclipsed
H
3
C Br
H
CH
3
H anti
H
CH
3
Br gauche
H
H
H
CH
3
Most stable
Total PE is 1.4 (H, CH
3
+ 3.8 (CH
3
ecl) + 1.0 (H,H ecl.)
, Br ecl.) = 6.2 kcal/mol total PE is 0.9 kcal/mol for
CH
3
/Br gauche
3. (10 pts) Menthol is a member of the terpene family of natural products. It exists naturally in a ( 1R , 2S , 5R ) form and a ( 1S , 2R , 5S ) form (ring numbering is shown). Draw the two molecules in their most stable chair conformer(s). Are these two compounds enantiomers or diastereomers ? Including the two natural forms, what is the maximum number of stereoisomers of menthol that are possible? _________
Draw flat molecules with wedge and dashes first—Simply use definition for multi-stereocenter molecules – if all stereocenters switch designation, the pair is enantiotopic
4. (9 pts) Name the following compounds using IPUAC nomenclature: a.) b.) c.) d.)
3-ethyl-3-methylhexane e.)
1-ethyl-3,3-dimethylcyclohexane f.)
3-methylbicyclo[3.2.1]octane
Br g.)
1-methyl-1-cycloheptene h.)
Trans-6,6-dimethyl-2-heptene
OH
OH
5-isopropyl-6-methyl-3-octanol cyclobutanol
5. (9 pts) Draw the following compounds: a.) isobutylcycloheptane b.) 3-methyl-2-octanol c.) 4,4-dimethyl-1-pentyne
4-bromo-1-pentyne d.) bicyclo[2.2.2]octane
OH e.) cis -1-iodo-2-butene
I
6. (10 pts) Assign R or S configurations to all of the chirality centers in the following pharmaceuticals/biomolecules:
OH H H
N
N
O S
Ephedrine – bronchodiolator and decongestant isolated
HO
N
H
Biotin – Vitamin B
7 from the Chinese plant ephedra sinica
7. (10 pts) The Pasteur separation of the mirror-image crystals of tartaric acid was one of the developments that led to the understanding of chirality in molecules. Draw all stereoisomers for tartaric acid and fully indicate the interrelationships of all (enantiomers, diastereomers or meso).
Be sure to assign R , S configurations to each chiral center.
O
HO
HO
O O
OH
OH
S S
R R
R S R S
8. (12 pts) Indicate whether the following pairs of compounds are identical, enantiomers, diastereomers or constitutional isomers.
a.
b.
c.
d.
Constitutional e.
Enantiomers f. identical
Diastereomers identical
identical
9. (5 pts) Compare the three staggered conformers of ethylene glycol. The anti conformation is not the lowest energy conformation. The other two staggered conformations are actually lower in energy than the anti conformation. Suggest an explanation.
In the other two staggered conformers, hydrogen bonding can occur between the hydroxyl groups; the formation of bonds lowers energy. When the hydroxyl groups are anti- this cannot occur.
H
H OH hydrogen bonding
H OH
H
10.
(5 pts) It is possible for a compound to be chiral even though it lacks a carbon atom with four different groups. For example, consider the structure of the following allene. Draw its enantiomer and explain why it is chiral.
H
3
C
H
C C C
CH
H
3
H
3
C
C
H
C C
CH
3
H
R, S-configurations are only a subset of chiral objects/molecules. The definition is essentially that two objects that are mirror images of one another are not superimposable. The mirror image of this allene is not superimposable on the original and therefore fits the definition of a chiral object.





